Drug Catalog - Product Detail
SODIUM SULFACETAMIDE WASH LIQ 0.1 16OZ
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 44523-0606-16 | BIOCOMP PHARMA | 480 | 10% | LIQUID |
PACKAGE FILES


Generic Name
SODIUM SULFACETAMIDE
Substance Name
SULFACETAMIDE SODIUM
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
Description
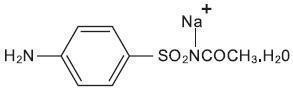
DESCRIPTION: Each gram contains 100 mg of sodium sulfacetamide in a vehicle consisting of: citric acid, disodium EDTA, fragrance, methylparaben, PEG-150 pentaerythrityl tetrastearate (and) aqua (and) PEG-6 caprylic/capric glycerides, PEG-60 almond triglycerides, propylene glycol, propylparaben, purified water, sodium chloride, and sodium laureth sulfate (and) cocamide DEA (and) cocamidopropyl betaine (and) glycol stearate. Sodium sulfacetamide is a sulfonamide with antibacterial activity. Sodium sulfacetamide is C 8 H 9 N 2 NaO 3 S·H 2 O with molecular weight of 254.24. Chemically, sodium sulfacetamide is N-[(4-aminophenyl) sulfonyl]-acetamide, monosodium salt, monohydrate. The structural formula is: Sodium sulfacetamide is an odorless, white, crystalline powder with a bitter taste. It is freely soluble in water, sparingly soluble in alcohol, while practically insoluble in benzene, in chloroform and in ether. structure
How Supplied
HOW SUPPLIED: This product is supplied in the following size(s): 12 fl. oz. (355 mL) bottles, NDC 44523-606-12 16 fl. oz. (480 mL) bottles, NDC 44523-606-16 To report a serious adverse event or obtain product information, call 1-866-762-2365. Manufactured for: BIOCOMP PHARMA ® , INC. San Antonio, TX 78230 1355 826177 I826177R0415
Indications & Usage
INDICATIONS: This product is intended for topical application in the following scaling dermatoses: seborrheic dermatitis and seborrhea sicca (dandruff). It also is indicated for the treatment of secondary bacterial infections of the skin due to organisms susceptible to sulfonamides.
Dosage and Administration
DOSAGE AND ADMINISTRATION: Seborrheic dermatitis including seborrhea sicca - Wash affected areas twice daily (morning and evening) or as directed by your physician. Avoid contact with eyes or mucous membranes. Wet skin and liberally apply to areas to be cleansed, massage gently into skin working into a full lather, rinse thoroughly, pat dry and repeat after 10 to 20 seconds. Rinsing with plain water will remove any excess medication. Repeat application as described above for 8 to 10 days or as directed by your physician. lf skin dryness occurs it may be controlled by rinsing cleanser off sooner or using less frequently. Regular shampooing following the use of this product is not necessary, but the hair should be shampooed at least once a week. As the condition subsides, the interval between applications may be lengthened. Applications once or twice weekly or every other week may prevent recurrence. Should the condition recur after stopping therapy, the application of this product should be reinitiated as at the beginning of treatment. Secondary cutaneous bacterial infections - Wash affected areas twice daily (morning and evening) or as directed by your physician. Avoid contact with eyes or mucous membranes. Wet skin and liberally apply to areas to be cleansed, massage gently into skin for 10 to 20 seconds working into a full lather, rinse thoroughly and pat dry. Rinsing with plain water will remove any excess medication. Repeat application as described above for 8 to 10 days or as directed by your physician. If skin dryness occurs it may be controlled by rinsing cleanser off sooner or using less frequently.
