Drug Catalog - Product Detail
SODIUM SULFACETAMIDE W/SULFUR SUSPENSION SUSP 10/5% 30GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 44523-0604-01 | BIOCOMP PHARMA | 30 | 10-5% | SUSPENSION |
PACKAGE FILES

Generic Name
SULFACETAMIDE SODIUM, SULFUR
Substance Name
SULFACETAMIDE SODIUM
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
Description
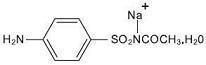
DESCRIPTION: Each gram contains 100 mg of sodium sulfacetamide and 50 mg of colloidal sulfur in a vehicle consisting of: benzyl alcohol, cetyl alcohol, disodium EDTA, fragrance, glyceryl stearate (and) PEG-100 stearate, magnesium aluminum silicate, PEG-150 distearate, phenoxyethanol, polyethylene glycol 400, purified water, sodium lauryl sulfate, sodium thiosulfate, stearyl alcohol and xanthan gum. Sodium sulfacetamide is a sulfonamide with antibacterial activity while sulfur acts as a keratolytic agent. Sodium sulfacetamide is C 8 H 9 N 2 NaO 3 S·H 2 O with molecular weight of 254.24. Chemically, sodium sulfacetamide is N-[(4-aminophenyl) sulfonyl]-acetamide, monosodium salt, monohydrate. The structural formula is: Sodium sulfacetamide is an odorless, white, crystalline powder with a bitter taste. It is freely soluble in water, sparingly soluble in alcohol, while practically insoluble in benzene, in chloroform and in ether. structure
How Supplied
HOW SUPPLIED: This product is supplied in the following size(s): 30 g bottles, NDC 44523-604-01 To report a serious adverse event or obtain product information, call 1-866-762-2365. Manufactured for: BIOCOMP PHARMA ® , INC. San Antonio, TX 78230 1355 L60401R1221
Indications & Usage
INDICATIONS: This product is indicated for use in the topical control of acne vulgaris, acne rosacea and seborrheic dermatitis.
Dosage and Administration
DOSAGE AND ADMINISTRATION: Shake well before using. Cleanse affected areas. Apply a thin layer to the affected areas with light massaging, 1 to 3 times daily or as directed by a physician.
