Drug Catalog - Product Detail
SORILUX CALCIPOTRIENE FOAM 0.005% 60GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 51862-0376-60 | MAYNE PHARMA | 60 | 0.005% | FOAM |
PACKAGE FILES







Generic Name
CALCIPOTRIENE
Substance Name
CALCIPOTRIENE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
NDA022563
Description
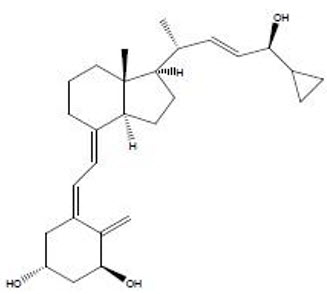
11 DESCRIPTION SORILUX Foam contains the compound calcipotriene, a synthetic vitamin D3 analog, in an aqueous-based emulsion foam vehicle for topical dermatologic use. Chemically, calcipotriene is (5Z,7E,22E,24S)-24-cyclopropyl-9,10-secochola-5,7,10(19), 22-tetraene- 1α,3β,24-triol. The structural formula is represented below: Molecular Formula: C27H40O3 Molecular Weight: 412.6 Calcipotriene is a white or off-white crystalline substance. SORILUX Foam contains calcipotriene 50 mcg/g in an aqueous-based emulsion foam vehicle consisting of cetyl alcohol, dibasic sodium phosphate, dl-α-tocopherol, edetate disodium, isopropyl myristate, light mineral oil, polyoxyl 20 cetostearyl ether, propylene glycol, purified water, stearyl alcohol, and white petrolatum. SORILUX Foam is dispensed from an aluminum can pressurized with a hydrocarbon (propane/n-butane/isobutane) propellant. Chemical Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied SORILUX (calcipotriene) Foam, 0.005%, is supplied as follows: 120 g aluminum can NDC 51862-376-12 16.2 Storage and Handling Store at 20ºC to 25°C (68°F to 77°F); excursions permitted to 15°C – 30°C (59°F – 86°F). Flammable. Contents under pressure. Do not puncture or incinerate. Do not expose to heat or store at temperatures above 120°F (49°C). Keep out of reach of children.
Indications & Usage
1 INDICATIONS AND USAGE SORILUX Foam is indicated for the topical treatment of plaque psoriasis of the scalp and body in adults and pediatric patients 4 years of age and older. SORILUX ® Foam is a vitamin D analog indicated for the topical treatment of plaque psoriasis of the scalp and body in adults and pediatric patients 4 years of age and older. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION SORILUX Foam is for topical use only. SORILUX Foam is not for oral, ophthalmic, or intravaginal use. Apply a thin layer of SORILUX Foam twice daily to the affected areas and rub in gently and completely. Avoid contact with the face and eyes. For topical use only; not for oral, ophthalmic, or intravaginal use. ( 2 ) Apply twice daily. ( 2 )
