Drug Catalog - Product Detail
Sulfamethoxazole & Trimethoprim 400mg-80mg Tablet 500
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0271-50 | AMNEAL PHARMACEUTICALS | 500 | 400-80MG | TABLET |
PACKAGE FILES


Generic Name
SULFAMETHOXAZOLE AND TRIMETHOPRIM
Substance Name
SULFAMETHOXAZOLE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA076899
Description
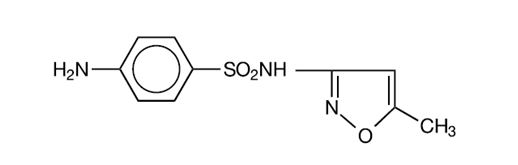
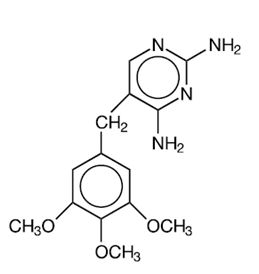
DESCRIPTION Sulfamethoxazole and trimethoprim is a synthetic antibacterial combination product available in DS (double strength) tablets, each containing 800 mg sulfamethoxazole, USP and 160 mg trimethoprim, USP; in tablets, each containing 400 mg sulfamethoxazole, USP and 80 mg trimethoprim, USP for oral administration. Sulfamethoxazole is N 1 -(5-methyl-3-isoxazolyl) sulfanilamide; the molecular formula is C 10 H 11 N 3 O 3 S. It is an almost white, odorless, tasteless compound with a molecular weight of 253.28 and the following structural formula: Trimethoprim is 2,4-diamino-5-(3,4,5-trimethoxybenzyl) pyrimidine; the molecular formula is C 14 H 18 N 4 O 3 . It is a white to light yellow, odorless, bitter compound with a molecular weight of 290.3 and the following structural formula: Inactive ingredients : Magnesium stearate, povidone, pregelatinized starch and sodium starch glycolate. lk uy
How Supplied
HOW SUPPLIED Sulfamethoxazole and Trimethoprim Tablets, USP are supplied as follows: Sulfamethoxazole and Trimethoprim DS (double strength) Tablets USP, 800 mg/160 mg, are supplied as white, oval, bisected tablets debossed “IP” bisect “272” on one side. They are available as follows: Bottles of 10: NDC 65162-272-01 Bottles of 24: NDC 65162-272-24 Bottles of 100: NDC 65162-272-10 Bottles of 250: NDC 65162-272-25 Bottles of 500: NDC 65162-272-50 Sulfamethoxazole and Trimethoprim Tablets USP, 400 mg/80 mg, are supplied as white, round, bisected tablets debossed “IP” over “271” on one side. They are available as follows: Bottles of 50: NDC 65162-271-05 Bottles of 100: NDC 65162-271-10 Bottles of 500: NDC 65162-271-50 Bottles of 1000: NDC 65162-271-11 Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. DISPENSE IN TIGHT, LIGHT-RESISTANT CONTAINER.
Indications & Usage
INDICATIONS AND USAGE To reduce the development of drug-resistant bacteria and maintain the effectiveness of sulfamethoxazole and trimethoprim tablets and other antibacterial drugs, sulfamethoxazole and trimethoprim tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to empiric selection of therapy. Urinary Tract Infections For the treatment of urinary tract infections due to susceptible strains of the following organisms: Escherichia coli , Klebsiella species, Enterobacter species, Morganella morganii , Proteus mirabilis and Proteus vulgaris . It is recommended that initial episodes of uncomplicated urinary tract infections be treated with a single effective antibacterial agent rather than the combination. Acute Otitis Media For the treatment of acute otitis media in pediatric patients due to susceptible strains of Streptococcus pneumoniae or Haemophilus influenzae when in the judgment of the physician sulfamethoxazole and trimethoprim offers some advantage over the use of other antimicrobial agents. To date, there are limited data on the safety of repeated use of sulfamethoxazole and trimethoprim tablets in pediatric patients under two years of age. Sulfamethoxazole and trimethoprim tablets are not indicated for prophylactic or prolonged administration in otitis media at any age. Acute Exacerbations of Chronic Bronchitis in Adults For the treatment of acute exacerbations of chronic bronchitis due to susceptible strains of Streptococcus pneumoniae or Haemophilus influenzae when a physician deems that sulfamethoxazole and trimethoprim could offer some advantage over the use of a single antimicrobial agent. Shigellosis For the treatment of enteritis caused by susceptible strains of Shigella flexneri and Shigella sonnei when antibacterial therapy is indicated. Pneumocystis jirovecii Pneumonia For the treatment of documented Pneumocystis jirovecii pneumonia and for prophylaxis against P. jirovecii pneumonia in individuals who are immunosuppressed and considered to be at an increased risk of developing P. jirovecii pneumonia. Traveler’s Diarrhea in Adults For the treatment of traveler’s diarrhea due to susceptible strains of enterotoxigenic E. coli .
Dosage and Administration
DOSAGE AND ADMINISTRATION Sulfamethoxazole and trimethoprim is contraindicated in pediatric patients less than 2 months of age. Urinary Tract Infections and Shigellosis in Adults and Pediatric Patients, and Acute Otitis Media in Children Adults: The usual adult dosage in the treatment of urinary tract infections is 1 sulfamethoxazole and trimethoprim double strength tablet or 2 sulfamethoxazole and trimethoprim tablets every 12 hours for 10 to 14 days. An identical daily dosage is used for 5 days in the treatment of shigellosis. Children: The recommended dose for children with urinary tract infections or acute otitis media is 40 mg/kg sulfamethoxazole and 8 mg/kg trimethoprim per 24 hours, given in two divided doses every 12 hours for 10 days. An identical daily dosage is used for 5 days in the treatment of shigellosis. The following table is a guideline for the attainment of this dosage: Children 2 months of age or older: Weight Dose – every 12 hours lb kg Tablets 22 10 - 44 20 1 66 30 1½ 88 40 2 or 1 DS tablet For Patients with Impaired Renal Function When renal function is impaired, a reduced dosage should be employed using the following table: Creatinine Clearance (mL/min) Recommended Dosage Regimen Above 30 Usual standard regimen 15 to 30 ½ the usual regimen Below 15 Use not recommended Acute Exacerbations of Chronic Bronchitis in Adults The usual adult dosage in the treatment of acute exacerbations of chronic bronchitis is 1 sulfamethoxazole and trimethoprim double strength tablet or 2 sulfamethoxazole and trimethoprim single strength tablets every 12 hours for 14 days. Pneumocystis Jirovecii Pneumonia Treatment Adults and Children The recommended dosage for treatment of patients with documented Pneumocystis jirovecii pneumonia is 75 mg/kg to 100 mg/kg sulfamethoxazole and 15 mg/kg to 20 mg/kg trimethoprim per 24 hours given in equally divided doses every 6 hours for 14 to 21 days 12 . The following table is a guideline for the upper limit of this dosage: Weight Dose – every 6 hours lb kg Tablets 18 8 - 35 16 1 53 24 1½ 70 32 2 or 1 DS tablet 88 40 2½ 106 48 3 or 1½ DS tablets 141 64 4 or 2 DS tablets 176 80 5 or 2½ DS tablets For the lower limit dose (75 mg/kg sulfamethoxazole and 15 mg/kg trimethoprim per 24 hours) administer 75% of the dose in the above table. Prophylaxis Adults: The recommended dosage for prophylaxis in adults is 1 sulfamethoxazole and trimethoprim double strength tablet daily 13 . Children: For children, the recommended dose is 750 mg/m 2 /day sulfamethoxazole with 150 mg/m 2 /day trimethoprim given orally in equally divided doses twice a day, on 3 consecutive days per week. The total daily dose should not exceed 1,600 mg sulfamethoxazole and 320 mg trimethoprim 14 . The following table is a guideline for the attainment of this dosage in children: Body Surface Area Dose – every 12 hours (m 2 ) Tablets 0.26 - 0.53 ½ 1.06 1 Traveler’s Diarrhea in Adults For the treatment of traveler’s diarrhea, the usual adult dosage is 1 sulfamethoxazole and trimethoprim double strength tablet or 2 sulfamethoxazole and trimethoprim single strength tablets every 12 hours for 5 days.
