Drug Catalog - Product Detail
TACLONEX SCALP CALCIPOTRIENE/BETAMETH DIP TOPICAL SUSPENSION 0.005-0.064% 60GM X 1
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 50222-0501-06 | LEO PHARMA INC | 60 | 0.005-0.064% | SUSPENSION |
PACKAGE FILES

Generic Name
CALCIPOTRIENE AND BETAMETHASONE DIPROPIONATE
Substance Name
BETAMETHASONE DIPROPIONATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
NDA022185
Description
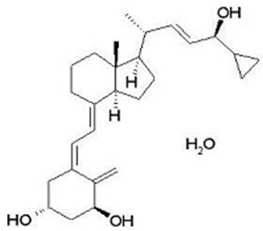
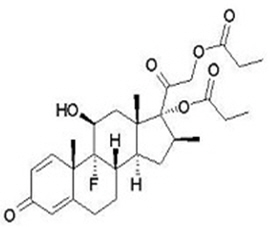
11 DESCRIPTION Taclonex Topical Suspension contains calcipotriene hydrate and betamethasone dipropionate. It is for topical use only. Calcipotriene hydrate is a synthetic vitamin D 3 analog. Calcipotriene Hydrate Calcipotriene hydrate is a vitamin D analog and has the chemical name 9,10-secochola-5,7,10(19),22-tetraene-1,3,24-triol,24-cyclo-propyl-,monohydrate, (1α,3β,5Z,7E,22E,24S) with the empirical formula C 27 H 40 O 3 ∙H 2 O, a molecular weight of 430.6, and the following structural formula (calcipotriene hydrate is a white to almost white, crystalline compound): Betamethasone Dipropionate Betamethasone dipropionate is a synthetic corticosteroid and has the chemical name pregna-1,4-diene-3,20-dione-9-fluoro-11-hydroxy-16-methyl-17,21- bis (1-oxypropoxy)-(11β,16β), with the empirical formula C 28 H 37 FO 7 , a molecular weight of 504.6, and the following structural formula (betamethasone dipropionate is a white to almost white, crystalline powder): Taclonex ® Topical Suspension Each gram of Taclonex Topical Suspension contains 50 mcg of calcipotriene (equivalent to 52.2 mcg of calcipotriene hydrate) and 0.643 mg of betamethasone dipropionate (equivalent to 0.5 mg of betamethasone) in a base of hydrogenated castor oil, polyoxypropylene stearyl ether, all- rac -alpha-tocopherol, butylhydroxytoluene, and mineral oil. Taclonex Topical Suspension is an odorless, clear to slightly off-white suspension. Chemical Structure Chemical Structure
How Supplied
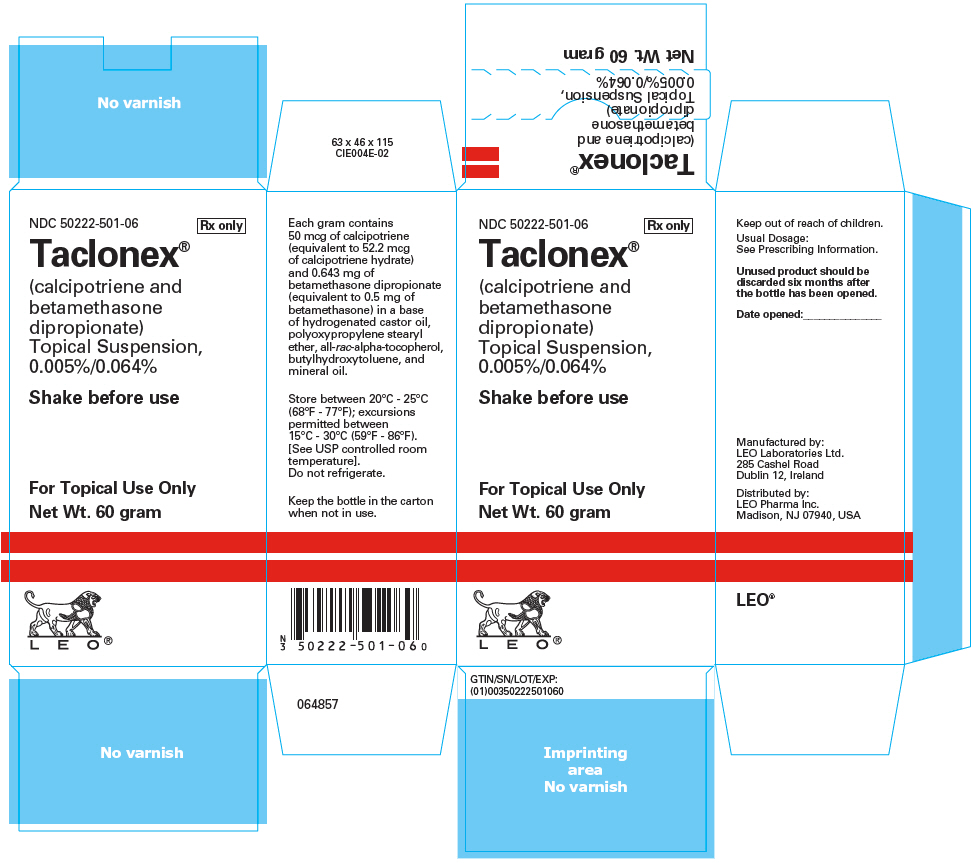
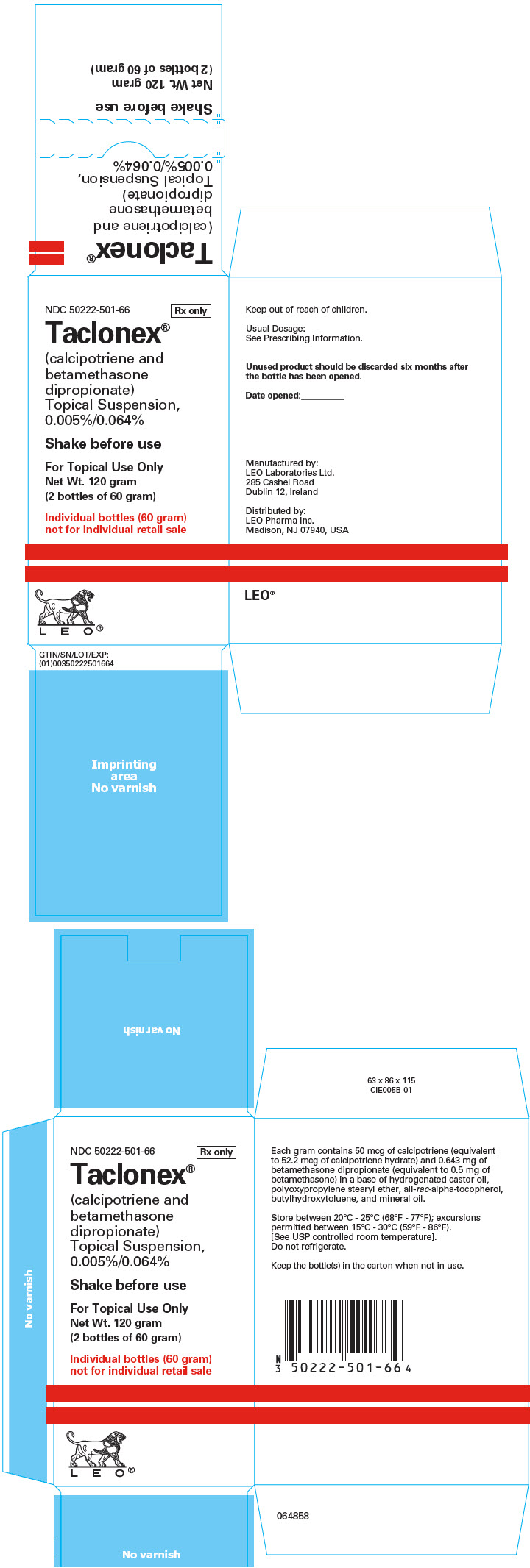
16 HOW SUPPLIED/STORAGE AND HANDLING Taclonex (calcipotriene and betamethasone dipropionate) Topical Suspension, 0.005%/0.064% is a viscous, nearly odorless, almost clear, colorless to slightly off-white suspension. It is available as: 60 gram bottle (NDC 50222-501-06) 120 gram (2 bottles of 60 gram) (NDC 50222-501-66) Store between 20°C - 25°C (68°F - 77°F); excursions permitted between 15°C - 30°C (59°F - 86°F). [See USP controlled room temperature]. Do not refrigerate. Keep the bottle in the carton when not in use. Unused product should be discarded six months after the bottle has been opened. Shake before use. Keep out of reach of children.
Indications & Usage
1 INDICATIONS AND USAGE Taclonex ® Topical Suspension is indicated for the topical treatment of plaque psoriasis of the scalp and body in patients 12 years and older. Taclonex Topical Suspension is a combination of calcipotriene, a vitamin D analog, and betamethasone dipropionate, a corticosteroid, indicated for the topical treatment of plaque psoriasis of the scalp and body in patients 12 years and older. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Instruct patients to shake bottle prior to using Taclonex Topical Suspension. Apply Taclonex Topical Suspension to affected areas on the scalp and body once daily for up to 8 weeks. Taclonex Topical Suspension should be discontinued when control is achieved. Instruct patients to wash their hands after applying the product. Inform patients that they should not take a bath or shower or wash their hair right after application of Taclonex Topical Suspension. Patients 12 to 17 years should not use more than 60 grams per week and patients 18 years and older should not use more than 100 grams per week. Taclonex Topical Suspension should not be: Used with occlusive dressings unless directed by a healthcare provider. Used on the face, groin, or axillae, or if skin atrophy is present at the treatment site. Applied to the scalp in the 12 hours before or after any chemical treatments to the hair. Taclonex Topical Suspension is not for oral, ophthalmic, or intravaginal use. Shake bottle before use. ( 2 ) Apply Taclonex Topical Suspension to affected areas on the scalp and body once daily for up to 8 weeks. Discontinue therapy when control is achieved. ( 2 ) Patients age 12 to 17 years should not use more than 60 grams per week.( 2 ) Adult patients should not use more than 100 grams per week. ( 2 ) Do not use with occlusive dressings unless directed by a healthcare provider. ( 2 ) Avoid use on the face, groin, or axillae, or if skin atrophy is present at the treatment site. ( 2 ) Not for oral, ophthalmic, or intravaginal use. ( 2 )
