Drug Catalog - Product Detail
TERCONAZOLE VAGINAL CREAM CRM 0.004 45GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00591-3196-89 | ACTAVIS PHARMA | 45GM | 0.4% | CREAM |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
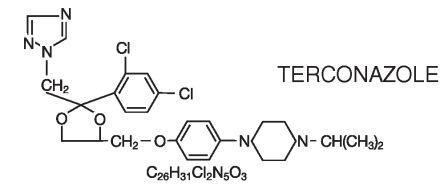
description Terconazole Vaginal Cream 0.4% is a white to off-white, water washable cream for intravaginal administration containing 0.4% of the antifungal agent terconazole, cis -1-[ p -[[2-(2,4-Dichlorophenyl)-2-(1 H -1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-isopropylpiperazine, compounded in a cream base consisting of butylated hydroxyanisole, cetyl alcohol, isopropyl myristate, polysorbate 60, polysorbate 80, propylene glycol, stearyl alcohol, and purified water. The structural formula of terconazole is as follows: Terconazole, a triazole derivative, is a white to almost white powder with a molecular weight of 532.47. It is insoluble in water; sparingly soluble in ethanol; and soluble in butanol. Chemical Structure for Terconazole
How Supplied
how supplied Terconazole Vaginal Cream 0.4% is available in 45 g (NDC 0591-3196-89) tubes with a measured-dose applicator. Store at Controlled Room Temperature 15° - 30° C (59° - 86° F). Terconazole Vaginal Cream 0.4% is available in 20 g (NDC 0591-3197-52) tubes with a measured-dose applicator. Store at Controlled Room Temperature 15° - 30° C (59° - 86° F).
Indications & Usage
indications and usage Terconazole Vaginal Cream is indicated for the local treatment of vulvovaginal candidiasis (moniliasis). As this product is effective only for vulvovaginitis caused by the genus Candida, the diagnosis should be confirmed by KOH smears and/or cultures.
Dosage and Administration
dosage and administration Terconazole Vaginal Cream 0.4%: One full applicator (5 g) of Terconazole Vaginal Cream (20 mg terconazole) should be administered intravaginally once daily at bedtime for seven consecutive days. Terconazole Vaginal Cream 0.4%: One full applicator (5 g) of Terconazole Vaginal Cream (40 mg terconazole) should be administered intravaginally once daily at bedtime for three consecutive days. Before prescribing another course of therapy, the diagnosis should be reconfirmed by smears and/or cultures and other pathogens commonly associated with vulvovaginitis ruled out. The therapeutic effect of these products is not affected by menstruation.
