Drug Catalog - Product Detail
TERCONAZOLE VAGINAL SUPPOSITORIES SUPP 80MG 3CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 45802-0717-08 | PADAGIS | 3 | 80MG | SUPPOSITORY |
PACKAGE FILES

Generic Name
TERCONAZOLE
Substance Name
TERCONAZOLE
Product Type
HUMAN PRESCRIPTION DRUG
Route
VAGINAL
Application Number
ANDA077149
Description
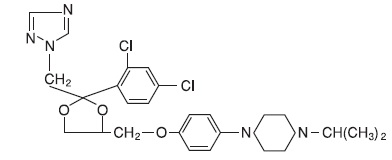
DESCRIPTION Terconazole Vaginal Suppositories, 80 mg are white to off-white suppositories for intravaginal administration containing 80 mg of the antifungal agent terconazole, cis -1-[ p -[[2-(2,4-Dichlorophenyl)-2- (1 H -1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-isopropylpiperazine, in triglycerides derived from coconut and/or palm kernel oil (a base of hydrogenated vegetable oils) and butylated hydroxyanisole. The structural formula of terconazole is as follows: Terconazole, a triazole derivative, is a white to almost white powder with a molecular weight of 532.47. It is insoluble in water; sparingly soluble in ethanol; and soluble in butanol. Structural Formula.jpg
How Supplied
HOW SUPPLIED Terconazole Vaginal Suppositories, 80 mg are available as follows: Carton containing 3 suppositories (NDC 45802- 717 -08)
Indications & Usage
INDICATIONS AND USAGE Terconazole Vaginal Suppositories, 80 mg are indicated for the local treatment of vulvovaginal candidiasis (moniliasis). As this product is effective only for vulvovaginitis caused by the genus Candida, the diagnosis should be confirmed by KOH smears and/or cultures.
Dosage and Administration
DOSAGE AND ADMINISTRATION One Terconazole Vaginal Suppository, 80 mg should be administered intravaginally once daily at bedtime for three consecutive days. Before prescribing another course of therapy, the diagnosis should be reconfirmed by smears and/or cultures and other pathogens commonly associated with vulvovaginitis ruled out. The therapeutic effect of terconazole vaginal suppositories is not affected by menstruation.
