Drug Catalog - Product Detail
TIAGABINE HCL 4MG 30CT TABS
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 62756-0224-83 | SUN PHARMACEUTICALS | 30 | 4MG | TABLET |
PACKAGE FILES


Generic Name
TIAGABINE HYDROCHLORIDE
Substance Name
TIAGABINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA077555
Description
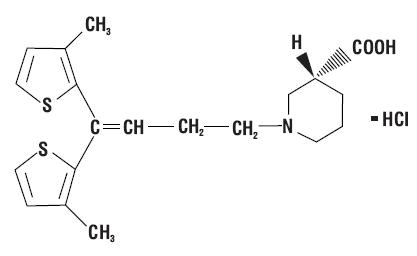
DESCRIPTION Tiagabine hydrochloride, USP is an antiepilepsy drug available as 2 mg and 4 mg tablets for oral administration. Its chemical name is (-)-(R)-1-[4,4-Bis(3- methyl-2-thienyl)-3-butenyl]nipecotic acid hydrochloride, its molecular formula is C 20 H 25 NO 2 S 2 HCl, and its molecular weight is 412. Tiagabine hydrochloride is a white to off-white, odorless, crystalline powder. It is insoluble in heptane, sparingly soluble in water, and soluble in aqueous base. The structural formula is: Inactive Ingredients Tiagabine hydrochloride tablets contain the following inactive ingredients: Hydrogenated vegetable oil, anhydrous lactose, butylated hydroxyanisole, pregelatinized maize starch, colloidal silicon dioxide, talc, crospovidone, titanium dioxide, hypromellose, polyethylene glycol. In addition, individual tablets contain: 2 mg tablets: FD&C Yellow No. 6 and polysorbate 80. 4 mg tablets: Lactose monohydrate and D&C Yellow No. 10. chemical-structure
How Supplied
HOW SUPPLIED Tiagabine hydrochloride tablets are available in two dosage strengths. 2 mg orange, circular, film-coated tablets debossed with “200” on one side and plain on the other side, are available in: Bottles of 30: Child Resistant Cap NDC 62756-200-83 Bottles of 1000: Non Child Resistant Cap NDC 62756-200-18 4 mg yellow, circular, film-coated tablets debossed with “224” on one side and plain on the other side, are available in: Bottles of 30: Child Resistant Cap NDC 62756-224-83 Bottles of 100: Child Resistant Cap NDC 62756-224-88 Non Child Resistant Cap NDC 62756-224-08 Bottles of 1000: Non Child Resistant Cap NDC 62756-224-18 Recommended Storage: Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C (59° and 86°F) [see USP Controlled Room Temperature]. Protect from light and moisture.
Indications & Usage
INDICATIONS AND USAGE Tiagabine hydrochloride tablets are indicated as adjunctive therapy in adults and children 12 years and older in the treatment of partial seizures.
Dosage and Administration
DOSAGE AND ADMINISTRATION General: The blood level of tiagabine obtained after a given dose depends on whether the patient also is receiving a drug that induces the metabolism of tiagabine. The presence of an inducer means that the attained blood level will be substantially reduced. Dosing should take the presence of concomitant medications into account. Tiagabine hydrochloride tablets are recommended as adjunctive therapy for the treatment of partial seizures in patients 12 years and older. The following dosing recommendations apply to all patients taking tiagabine hydrochloride tablets: Tiagabine hydrochloride tablets are given orally and should be taken with food. Do not use a loading dose of tiagabine hydrochloride tablets Dose titration: Rapid escalation and/or large dose increments of tiagabine hydrochloride tablets should not be used. Missed dose(s): If the patient forgets to take the prescribed dose of tiagabine hydrochloride tablets at the scheduled time, the patient should not attempt to make up for the missed dose by increasing the next dose. If a patient has missed multiple doses, patient should refer back to his or her physician for possible re-titration as clinically indicated. Dosage adjustment of tiagabine hydrochloride tablets should be considered whenever a change in patient's enzyme-inducing status occurs as a result of the addition, discontinuation, or dose change of the enzyme-inducing agent. Induced Adults and Adolescents 12 Years or Older: The following dosing recommendations apply to patients who are already taking enzyme-inducing antiepilepsy drugs (AEDs) (e.g., carbamazepine, phenytoin, primidone, and phenobarbital). Such patients are considered induced patients when administering tiagabine hydrochloride tablets. In adolescents 12 to 18 years old, tiagabine hydrochloride tablets should be initiated at 4 mg once daily. Modification of concomitant antiepilepsy drugs is not necessary, unless clinically indicated. The total daily dose of tiagabine hydrochloride tablets may be increased by 4 mg at the beginning of Week 2. Thereafter, the total daily dose may be increased by 4 to 8 mg at weekly intervals until clinical response is achieved or up to 32 mg/day. The total daily dose should be given in divided doses two to four times daily. Doses above 32 mg/day have been tolerated in a small number of adolescent patients for a relatively short duration. In adults, tiagabine hydrochloride tablets should be initiated at 4 mg once daily. Modification of concomitant antiepilepsy drugs is not necessary, unless clinically indicated. The total daily dose of tiagabine hydrochloride tablets may be increased by 4 to 8 mg at weekly intervals until clinical response is achieved or, up to 56 mg/day. The total daily dose should be given in divided doses two to four times daily. Doses above 56 mg/day have not been systematically evaluated in adequate and well-controlled clinical trials. Experience is limited in patients taking total daily doses above 32 mg/day using twice daily dosing. A typical dosing titration regimen for patients taking enzyme-inducing AEDs (induced patients) is provided in Table 7 . Table 7: Typical Dosing Titration Regimen for Patients Already Taking Enzyme-Inducing AEDs Initiation and Titration Schedule Total Daily Dose Week 1 Initiate at 4 mg once daily 4 mg/day Week 2 Increase total daily dose by 4 mg 8 mg/day (in two divided doses) Week 3 Increase total daily dose by 4 mg 12 mg/day (in three divided doses) Week 4 Increase total daily dose by 4 mg 16 mg/day (in two to four divided doses) Week 5 Increase total daily dose by 4 to 8 mg 20 to 24 mg/day (in two to four divided doses) Week 6 Increase total daily dose by 4 to 8 mg 24 to 32 mg/day (in two to four divided doses) Usual Adult Maintenance Dose in Induced Patients: 32 to 56 mg/day in two to four divided doses Non-Induced Adults and Adolescents 12 Years or Older: The following dosing recommendations apply to patients who are taking only non-enzyme-inducing AEDs. Such patients are considered non-induced patients: Following a given dose of tiagabine hydrochloride tablets, the estimated plasma concentration in the non-induced patients is more than twice that in patients receiving enzyme-inducing agents. Use in non-induced patients requires lower doses of tiagabine hydrochloride tablets. These patients may also require a slower titration of tiagabine hydrochloride tablets compared to that of induced patients (see CLINICAL PHARMACOLOGY, Pharmacokinetics and PRECAUTIONS, General, Use in Non-Induced Patients ).
