Drug Catalog - Product Detail
TIMOLOL MALCTTE OPHTH GEL FORMING SOLN 0.25% 5 ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 24208-0818-25 | BAUSCH HEALTH | 5 | 0.25% | SOLUTION |
PACKAGE FILES
Generic Name
TIMOLOL MALEATE
Substance Name
TIMOLOL MALEATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
OPHTHALMIC
Application Number
NDA020330
Description
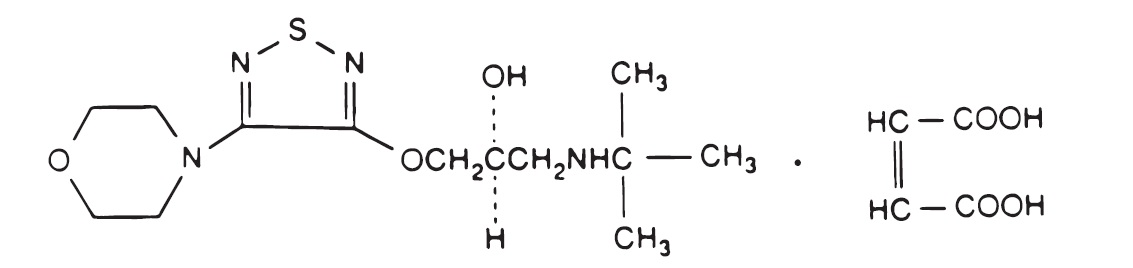
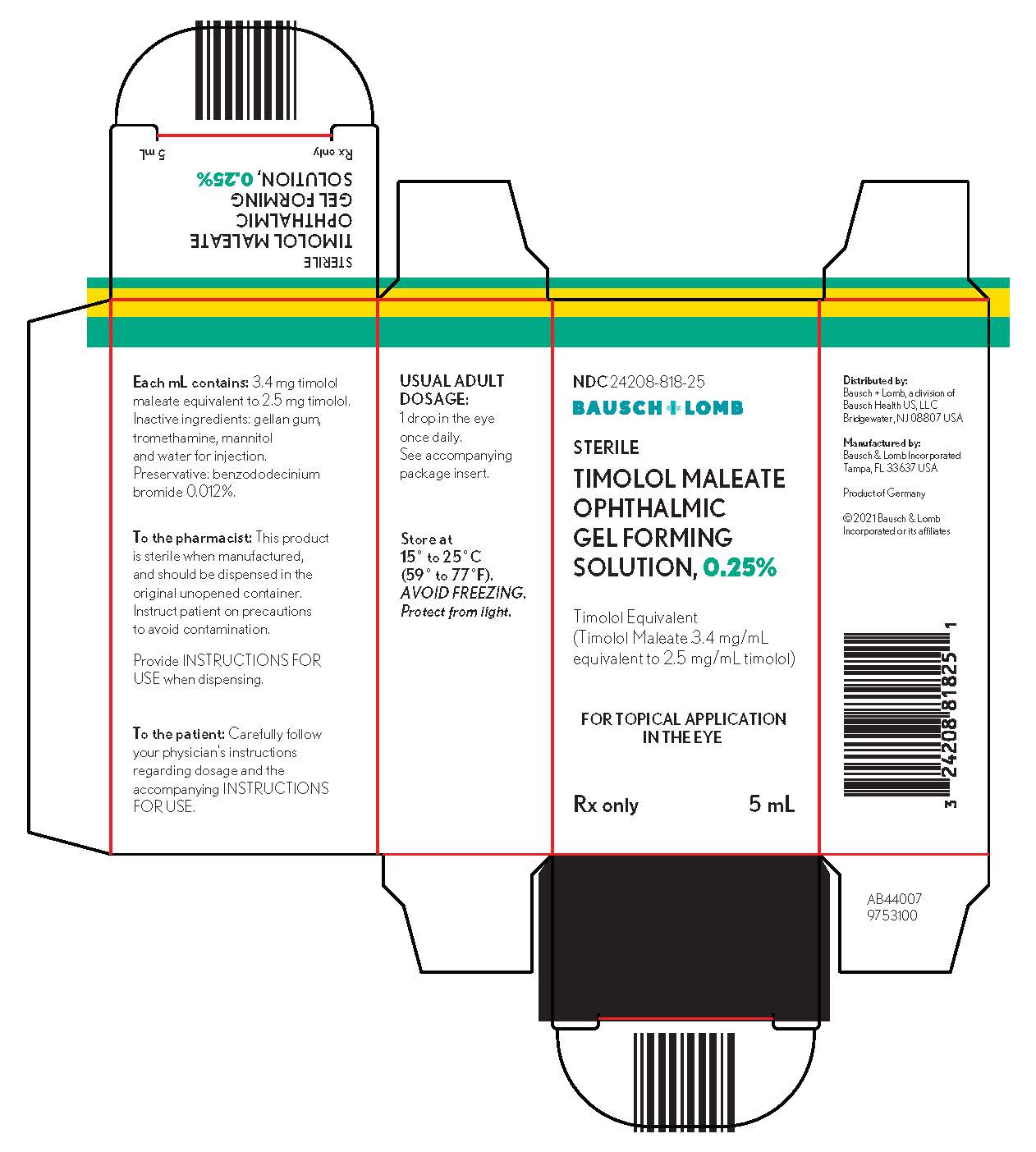
DESCRIPTION Timolol Maleate Ophthalmic Gel Forming Solution is a non-selective beta-adrenergic receptor blocking agent. Its chemical name is (-)-1-( tert -butylamino)-3-[(4-morpholino-1,2,5-thiadiazol-3-yl)oxy]-2-propanol maleate (1:1) (salt). Timolol maleate possesses an asymmetric carbon atom in its structure and is provided as the levo-isomer. The optical rotation of timolol maleate is: 25° [α] in 1.0N HCl (C = 5%) = -12.2° (-11.7° to -12.5°). 405 nm Its molecular formula is C 13 H 24 N 4 O 3 S•C 4 H 4 O 4 and its structural formula is: Timolol maleate has a molecular weight of 432.50. It is a white, odorless, crystalline powder which is soluble in water, methanol, and alcohol. Timolol Maleate Sterile Ophthalmic Gel Forming Solution is supplied as a sterile, isotonic, buffered, aqueous solution of timolol maleate in two dosage strengths. The pH of the solution is approximately 7.0, and the osmolarity is 260-330 mOsm. Each mL of Timolol Maleate Ophthalmic Gel Forming Solution 0.25% contains 2.5 mg of timolol (3.4 mg of timolol maleate). Each mL of Timolol Maleate Ophthalmic Gel Forming Solution 0.5% contains 5 mg of timolol (6.8 mg of timolol maleate). Inactive ingredients: gellan gum, tromethamine, mannitol, and water for injection. Preservative: benzododecinium bromide 0.012%. The gel forming solution contains a purified anionic heteropolysaccharide derived from gellan gum. An aqueous solution of gellan gum, in the presence of a cation, has the ability to gel. Upon contact with the precorneal tear film, Timolol Maleate Ophthalmic Gel Forming Solution forms a gel that is subsequently removed by the flow of tears. CHEM
How Supplied
HOW SUPPLIED Timolol Maleate Ophthalmic Gel Forming Solution is a colorless to nearly colorless, slightly opalescent, and slightly viscous solution. Timolol Maleate Ophthalmic Gel Forming Solution, 0.25% timolol equivalent, is supplied in a white low density polyethylene (LDPE) dispenser with a controlled drop tip and a yellow polypropylene cap as follows: NDC 24208-818-25 , 5 mL in a 7.5 mL capacity bottle. Timolol Maleate Ophthalmic Gel Forming Solution, 0.5% timolol equivalent, is supplied in a white low density polyethylene (LDPE) dispenser with a controlled drop tip and a yellow polypropylene cap as follows: NDC 24208-819-05 , 5 mL in a 7.5 mL capacity bottle. Storage Store at 15° to 25°C (59° to 77°F). Avoid Freezing. Protect from light. Distributed by: Bausch + Lomb, a division of Bausch Health US, LLC Bridgewater, NJ 08807 USA Manufactured by: Bausch & Lomb Incorporated Tampa, FL 33637 USA © 2021 Bausch & Lomb Incorporated or its affiliates Revised: 02/2021 9753400
Indications & Usage
INDICATIONS AND USAGE Timolol Maleate Sterile Ophthalmic Gel Forming Solution is indicated in the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma.
Dosage and Administration
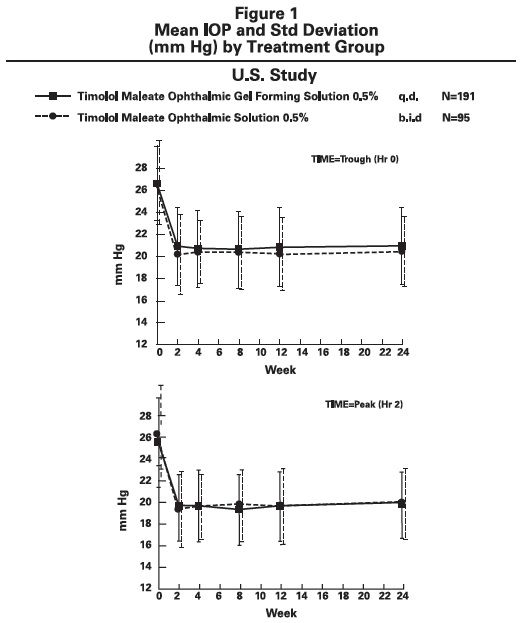
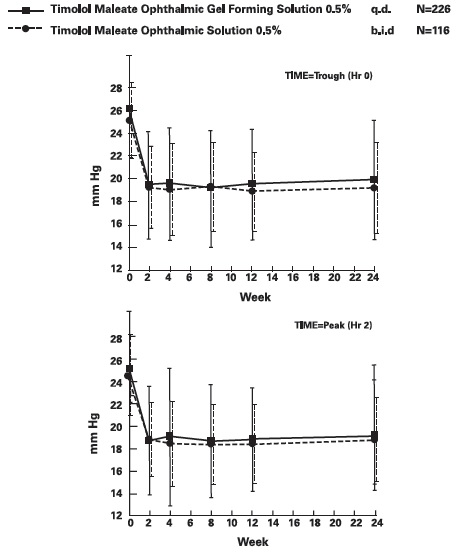
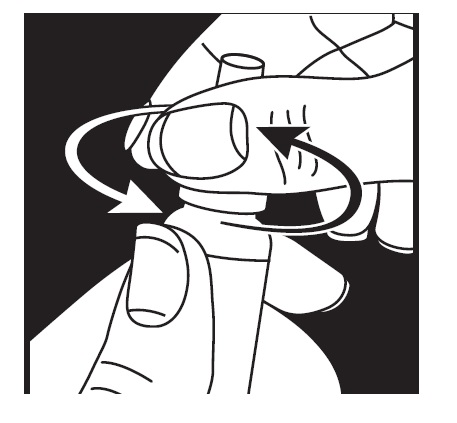
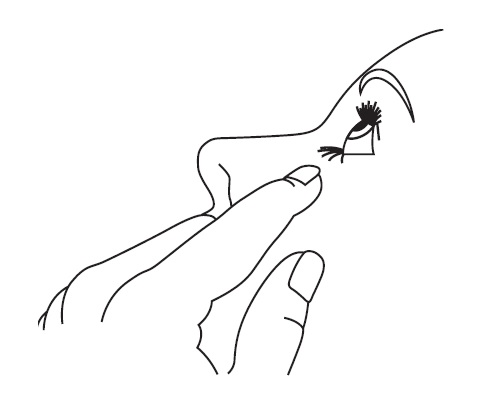
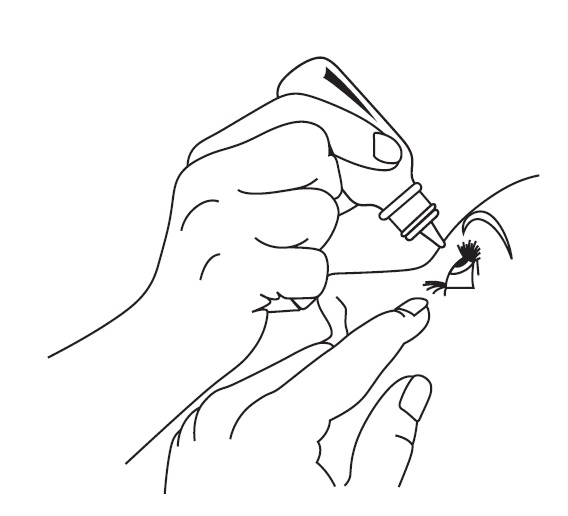
DOSAGE AND ADMINISTRATION Patients should be instructed to invert the closed container and shake once before each use. It is not necessary to shake the container more than once. Other topically applied ophthalmic medications should be administered at least 10 minutes before Timolol Maleate Ophthalmic Gel Forming Solution [see PRECAUTIONS , Information for Patients and accompanying INSTRUCTIONS FOR USE ]. Timolol Maleate Sterile Ophthalmic Gel Forming Solution is available in concentrations of 0.25% and 0.5%. The dose is one drop of Timolol Maleate Ophthalmic Gel Forming Solution (either 0.25% or 0.5%) in the affected eye(s) once a day. Because in some patients the pressure-lowering response to Timolol Maleate Ophthalmic Gel Forming Solution may require a few weeks to stabilize, evaluation should include a determination of intraocular pressure after approximately 4 weeks of treatment with Timolol Maleate Ophthalmic Gel Forming Solution. Dosages higher than one drop of 0.5% Timolol Maleate Ophthalmic Gel Forming Solution once a day have not been studied. If the patient’s intraocular pressure is still not at a satisfactory level on this regimen, concomitant therapy can be considered. The concomitant use of two topical beta-adrenergic blocking agents is not recommended [see PRECAUTIONS , Drug Interactions , Beta-Adrenergic Blocking Agents ]. When patients have been switched from therapy with Timolol Maleate Ophthalmic Solution administered twice daily to Timolol Maleate Ophthalmic Gel Forming Solution administered once daily, the ocular hypotensive effect has remained consistent.
