Drug Catalog - Product Detail
TIMOLOL MALEATE OPHTH SOL 0.005 10ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 17478-0288-11 | AKORN | 10 | 0.5% | SOLUTION |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
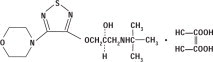
DESCRIPTION Timolol maleate ophthalmic solution USP is a non-selective beta-adrenergic receptor blocking agent. Its chemical name is (-)-1-( tert -butylamino)-3-[(4-morpholino-1,2,5-thiadiazol-3-yl)-oxy]-2-propanol maleate (1:1) (salt). Timolol maleate possesses an asymmetric carbon atom in its structure and is provided as the levo-isomer. The nominal optical rotation of timolol maleate is: 25° [α] in 1.0N HCl (C = 5%) = -12.2° (-11.7° to -12.5°) 405 nm Its molecular formula is C 13 H 24 N 4 O 3 S•C 4 H 4 O 4 and its structural formula is: Timolol maleate has a molecular weight of 432.50. It is a white, odorless, crystalline powder which is soluble in water, methanol, and alcohol. Timolol maleate ophthalmic solution is stable at room temperature. Timolol Maleate Ophthalmic Solution is supplied as a sterile, isotonic, buffered, aqueous solution of timolol maleate in two dosage strengths: Timolol Maleate Ophthalmic Solution, 0.25% Each mL contains: Active: timolol maleate 3.4 mg, equivalent to 2.5 mg timolol (0.25%). Inactives: dibasic sodium phosphate, monobasic sodium phosphate, sodium hydroxide to adjust pH and Water for Injection, USP. The pH of the solution is approximately 7.0, and the osmolarity is 274-328 mOsm. Preservative: benzalkonium chloride 0.1 mg (0.01%). Timolol Maleate Ophthalmic Solution, 0.5% Each mL contains: Active: timolol maleate 6.8 mg, equivalent to 5 mg timolol (0.5%). Inactives: dibasic sodium phosphate, monobasic sodium phosphate, sodium hydroxide to adjust pH and Water for Injection, USP. Preservative: benzalkonium chloride 0.1 mg (0.01%). Structural Formula
How Supplied
HOW SUPPLIED Timolol Maleate Ophthalmic Solution is a clear, colorless to light yellow solution. Timolol Maleate Ophthalmic Solution, 0.25% timolol equivalent, is supplied in a white opaque, plastic ophthalmic bottle with a controlled drop tip as follows: 2.5 mL, 5 mL, 10 mL and 15 mL. NDC 17478-289-25 2.5 mL NDC 17478-289-10 5 mL NDC 17478-289-11 10 mL NDC 17478-289-12 15 mL Timolol Maleate Ophthalmic Solution, 0.5% timolol equivalent, is supplied in a white opaque, plastic ophthalmic bottle with a controlled drop tip as follows: 2.5 mL, 5 mL, 10 mL and 15 mL. NDC 17478-288-25 2.5 mL NDC 17478-288-10 5 mL NDC 17478-288-11 10 mL NDC 17478-288-12 15 mL STORAGE Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from freezing. Protect from light. AKORN Manufactured By: Akorn, Inc. Lake Forest, IL 60045 TL00NS Rev. 08/16
Indications & Usage
INDICATIONS AND USAGE Timolol Maleate Ophthalmic Solution is indicated in the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma.
Dosage and Administration
DOSAGE AND ADMINISTRATION Timolol Maleate Ophthalmic Solution is available in concentrations of 0.25 and 0.5%. The usual starting dose is one drop of 0.25% timolol in the affected eye(s) twice a day. If the clinical response is not adequate, the dosage may be changed to one drop of 0.5% solution in the affected eye(s) twice a day. Since in some patients the pressure-lowering response to timolol may require a few weeks to stabilize, evaluation should include a determination of intraocular pressure after approximately 4 weeks of treatment with timolol. If the intraocular pressure is maintained at satisfactory levels, the dosage schedule may be changed to one drop once a day in the affected eye(s). Because of diurnal variations in intraocular pressure, satisfactory response to the once-a-day dose is best determined by measuring the intraocular pressure at different times during the day. Dosages above one drop of 0.5% timolol twice a day generally have not been shown to produce further reduction in intraocular pressure. If the patient's intraocular pressure is still not at a satisfactory level on this regimen, concomitant therapy with other agent(s) for lowering intraocular pressure can be instituted. The concomitant use of two topical beta-adrenergic blocking agents is not recommended (see PRECAUTIONS, Drug Interactions, Beta-adrenergic blocking agents ).
