Drug Catalog - Product Detail
Tizanidine HCl Cap 6 MG Base Equivalent 150 EA
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 75834-0209-15 | NIVAGEN PHARMACEUTICALS | 150 | 6MG | CAPSULE |
PACKAGE FILES



Generic Name
TIZANIDINE HYDROCHLORIDE
Substance Name
TIZANIDINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA210021
Description
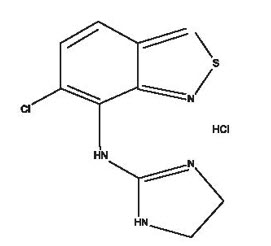
11 DESCRIPTION Tizanidine hydrochloride is a central alpha 2 -adrenergic agonist. Tizanidine HCl is a white to slight⍰ yellow crystalline powder. Tizanidine is soluble in water and methanol. Its chemical name is 5-chloro-4-(2-imidazolin-2-ylamino)-2, 1 ,3-benzothiadiazole hydrochloride. Tizanidine's molecular formula is C 9 H 8 CIN 5 S-HCl, its molecular weight is 290.2 and its structural formula is: Tizanidine hydrochloride capsules are supplied as 2, 4, and 6 mg capsules for oral administration. Tizanidine hydrochloride capsules contain the active ingredient, tizanidine hydrochloride (2.29 mg equivalent to 2 mg tizanidine base, 4.58 mg equivalent to 4 mg tizanidine base, and 6.87 mg equivalent to 6 mg tizanidine base), and the inactive ingredients, lactose monohydrate, hypromellose, microcrystalline cellulose and colloidal silicon dioxide. Each capsule shell contains FD & C Blue t, FD & C Red 3, titanium dioxide and gelatin. The capsules are printed with SW-0012 while ink which contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, potassium hydroxide, and titanium dioxide. Chemical Structure
How Supplied
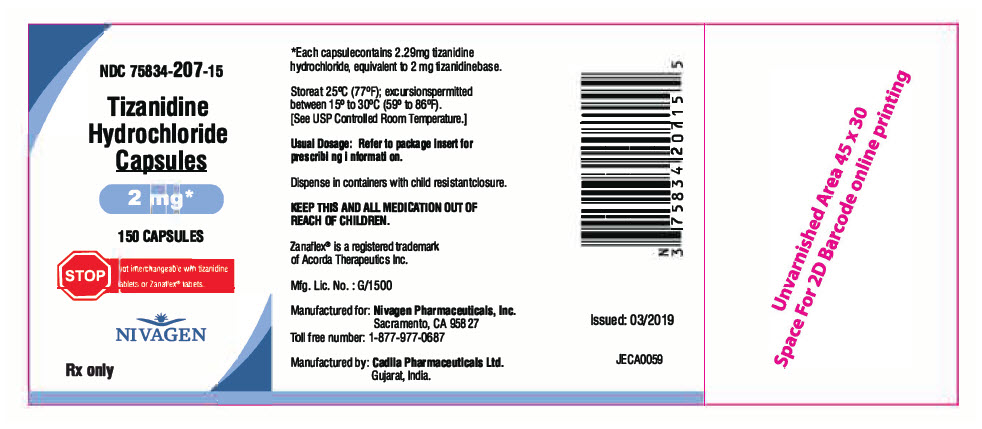
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 Tizanidine Hydrochloride Capsules Tizanidine hydrochloride capsules are available in three strengths as two-piece hard gelatin capsules containing tizanidine hydrochloride 2.29 mg, 4.58 mg and 6.87 mg, equivalent to 2 mg, 4 mg and 6 mg tizanidine base. The 2 mg capsules have a light yellow powder filled in size "3" hard gelatin capsule with opaque light blue cap imprinted "2 MG" and opaque light blue body: bottle of 150 capsules (NDC 75834-207-15) The 4 mg capsules have a light yellow powder filled in size "3" hard gelatin capsule with opaque blue cap imprinted "4 MG" and opaque white body: bottle of 150 capsules (NDC 75834-208-15) The 6 mg capsules have a light yellow powder filled in size "2" hard gelatin capsule with opaque blue cap imprinted "6 MG" and opaque blue body: bottle of 150 capsules (NDC 75834-209-15) Store at 25°G (77°F); excursions permitted between 15° to 30°G (59° to 86°F). [See USP Controlled Room Temperature]. Dispense in containers with child resistant closure.
Indications & Usage
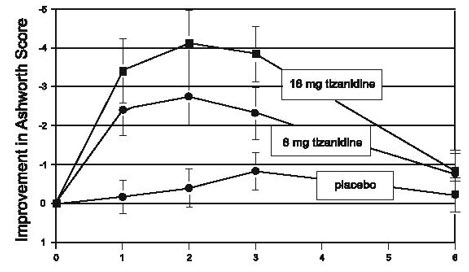
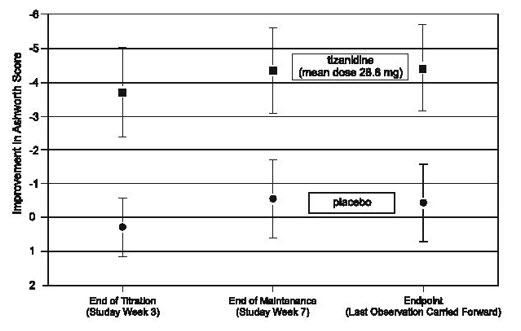
1 INDICATIONS AND USAGE Tizanidine is a central alpha-2-adrenergic agonist indicated for the management of spasticity. Because of the short duration of therapeutic effect, treatment with tizanidine should be reserved for those daily activities and times when relief of spasticity is most important [see Dosage and Administration (2.1) ]. Tizanidine is a central alpha-2-adrenergic agonist indicated for the management of spasticity. Because of the short duration of therapeutic effect, treatment with tizanidine should be reserved for those daily activities and times when relief of spasticity is most important. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Recommended starting dose: 2 mg; dose can be repeated at 6 to 8 hour intervals, up to a maximum of 3 doses in 24 hours ( 2.1 ) Dosage can be increased by 2 mg to 4 mg per dose, wHh 1 to 4 days between increases; total daily dose should not exceed 36 mg ( 2.1 ) Tizanidine pharmacokinetics differs between tablets and capsules, and when taken with or without food. These differences could result in a change in tolerability and control of symptoms ( 2.1 , 12.3 ) To discontinue tizanidine, decrease dose slowly to minimize the risk of withdrawal and rebound hypertension, tachycardia, and hypertonia ( 2.2 ) 2.1 Dosing lnformation Tizanidine hydrochloride capsules may be prescribed with or without food. Once the formulation has been selected and the decision to take with or without food has been made, this regimen should not be altered. Food has complex effects on tizanidine pharmacokinetics, which differ with the different formulations. Tizanidine hydrochloride capsules and tizanidine hydrochloride tablets are bioequivalent to each other under fasting conditions (more than 3 hours after a meal), but not under fed conditions (within 30 minutes of a meal). These pharmacokinetic differences may result in clinically significant differences when switching administration of tablet and capsules and when switching administration between the fed or fasted state. These changes may result in increased adverse events, or delayed or more rapid onset of activity, depending upon the nature of the switch. For this reason, the prescriber should be thoroughly familiar with the changes in kinetics associated with these different conditions [see Clinical Pharmacology (12.3) ]. The recommended starting dose is 2 mg. Because the effect of tizanidine peaks at approximately 1 to 2 hours post-dose and dissipates between 3 to 6 hours post-dose, treatment can be repeated at 6 to 8 hour intervals, as needed, to a maximum of three doses in 24 hours. Dosage can be gradually increased by 2 mg to 4 mg at each dose, with 1 to 4 days between dosage increases, until a satisfactory reduction of muscle tone is achieved. The total daily dose should not exceed 36 mg. Single doses greater than 16 mg have not been studied. 2.2 Dosing in Patients with Renal Impairment Tizanidine should be used with caution in patients with renal insufficiency (creatinine clearance < 25 ml/min), as clearance is reduced by more than 50%. In these patients, during titration, the individual doses should be reduced. 11 higher doses are required, individual doses rather than dosing frequency should be increased [see Warnings and Precautions (5. 7) ]. 2.3 Dosing in Patients with Hepatic Impairment Tizanidine should be used with caution in patients with any hepatic impairment. In these patients, during titration, the individual doses should be reduced. If higher doses are required, individual doses rather than dosing frequency should be increased. Monitoring of aminotransferase levels is recommended for baseline and 1 month after maximum dose is achieved, or if hepatic injury is suspected. [see Use in Specific Populations (8. 7) ]. 2.4 Drug Discontinuation If therapy needs to be discontinued, particularly in patients who have been receiving high doses (20 mg to 36 mg daily) for long periods (9 weeks or more) or who may be on concomitant treatment with narcotics, the dose should be decreased slowly (2 mg to 4 mg per day) to minimize the risk of withdrawal and rebound hypertension, tachycardia, and hypertonia [see Drug Abuse and Dependence (9.3) ].
