Drug Catalog - Product Detail
TOBRAMYCIN/DEXAMETHASONE OPHTH. SUSPENSION SUSP 0.3/0.1% 10ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 61314-0647-10 | SANDOZ | 10 | 0.3-0.1% | SUSPENSION |
PACKAGE FILES


Generic Name
TOBRAMYCIN AND DEXAMETHASONE
Substance Name
DEXAMETHASONE
Product Type
HUMAN PRESCRIPTION DRUG
Route
OPHTHALMIC
Application Number
NDA050592
Description
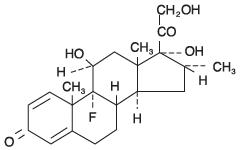
DESCRIPTION Tobramycin and dexamethasone ophthalmic suspension is a sterile, multiple dose antibiotic and steroid combination for topical ophthalmic use. The chemical structures for tobramycin and dexamethasone are presented below: Tobramycin Empirical Formula: C 18 H 37 N 5 O 9 Chemical Name: O -3-Amino-3-deoxy-α- D-glucopyranosyl-(1→4)- O -[2,6-diamino-2,3,6-trideoxy-α-D- ribo -hexopyranosyl- (1→6)]-2-deoxy-L-streptamine Molecular Weight: 467.52 Dexamethasone Empirical Formula: C 22 H 29 FO 5 Chemical Name: 9-Fluoro-11β,17,21-trihydroxy-16α-methylpregna-1,4-diene-3,20-dione Molecular Weight: 392.47 Each mL of tobramycin and dexamethasone ophthalmic suspension contains: Actives: tobramycin 0.3% (3 mg) and dexamethasone 0.1% (1 mg). Preservative: benzalkonium chloride 0.01%. Inactives: edetate disodium, hydroxyethyl cellulose, purified water, sodium chloride, sodium sulfate, sulfuric acid and/or sodium hydroxide (to adjust pH), and tyloxapol. tobramycin dexamethasone
How Supplied
HOW SUPPLIED Sterile ophthalmic suspension in 2.5 mL (NDC61314-647-25), 5 mL (NDC61314-647-05) and 10 mL (NDC61314-647-10) dispensers. STORAGE Store at 8°C to 27°C (46°F to 80°F). Store suspension upright and shake well before using. After opening, tobramycin and dexamethasone ophthalmic suspension can be used until the expiration date on the bottle. Distributed by Sandoz Inc. Princeton, NJ 08540 May 2021 T2021-123 775950 US
Indications & Usage
INDICATIONS AND USAGE Tobramycin and dexamethasone ophthalmic suspension is indicated for steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and where superficial bacterial ocular infection or a risk of bacterial ocular infection exists. Ocular steroids are indicated in inflammatory conditions of the palpebral and bulbar conjunctiva, cornea and anterior segment of the globe where the inherent risk of steroid use in certain infective conjunctivitides is accepted to obtain a diminution in edema and inflammation. They are also indicated in chronic anterior uveitis and corneal injury from chemical, radiation or thermal burns, or penetration of foreign bodies. The use of a combination drug with an anti-infective component is indicated where the risk of superficial ocular infection is high or where there is an expectation that potentially dangerous numbers of bacteria will be present in the eye. The particular anti-infective drug in this product is active against the following common bacterial eye pathogens: Staphylococci, including S. aureus and S. epidermidis (coagulase-positive and coagulase-negative), including penicillin-resistant strains. Streptococci, including some of the Group A-beta-hemolytic species, some nonhemolytic species, and some Streptococcus pneumoniae . Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Proteus mirabilis, Morganella morganii, most Proteus vulgaris strains, Haemophilus influenzae and H. aegyptius, Moraxella lacunata, Acinetobacter calcoaceticus and some Neisseria species.
Dosage and Administration
DOSAGE AND ADMINISTRATION One or two drops instilled into the conjunctival sac(s) every four to six hours. During the initial 24 to 48 hours, the dosage may be increased to one or two drops every two (2) hours. Frequency should be decreased gradually as warranted by improvement in clinical signs. Care should be taken not to discontinue therapy prematurely. Not more than 20 mL should be prescribed initially and the prescription should not be refilled without further evaluation as outlined in PRECAUTIONS above.
