Drug Catalog - Product Detail
TRANDOLAPRIL TB 4MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68180-0568-01 | LUPIN PHARMACEUTICALS | 100 | 4MG | TABLET |
PACKAGE FILES



Generic Name
TRANDOLAPRIL
Substance Name
TRANDOLAPRIL
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA077522
Description
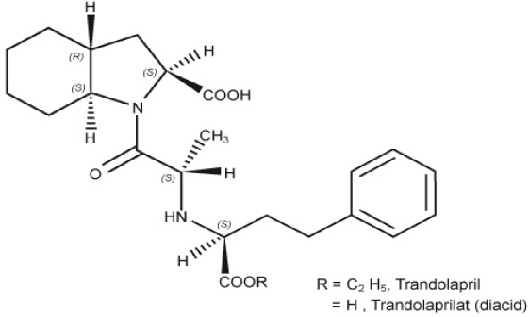
DESCRIPTION Trandolapril is the ethyl ester prodrug of a nonsulfhydryl angiotensin converting enzyme (ACE) inhibitor, trandolaprilat. Trandolapril is chemically described as (2S,3aR,7aS)-1-[(S)-N-[(S)-1-Carboxy-3-phenylpropyl] alanyl] hexahydro-2-indolinecarboxylic acid, 1-ethyl ester. Its empirical formula is C 24 H 34 N 2 O 5 and its structural formula is Image M.W.=430.54 Melting Point=125°C Trandolapril is a white or almost white powder that is soluble (>100 mg/mL) in chloroform, dichloromethane, and methanol. Trandolapril tablets USP contain 1 mg, 2 mg, or 4 mg of trandolapril for oral administration. Each tablet also contains croscarmellose sodium, hypromellose, lactose monohydrate, povidone, starch (corn starch), sodium stearyl fumarate, red iron oxide or yellow iron oxide. image
How Supplied
HOW SUPPLIED Trandolapril tablets USP are supplied as follows: 1 mg tablet - Pink, round, biconvex, uncoated tablets, debossed with 'L' and 'U' on either side of the breakline on one side and 'H01' on the other side. NDC 68180-566-01 - bottles of 100 2 mg tablet - Yellow, round, biconvex, uncoated tablets, debossed with 'LU' on one side and 'H02' on the other side. NDC 68180-567-01 - bottles of 100 4 mg tablet - Brick red colored, round, biconvex, uncoated tablets, debossed with 'LU' on one side and 'H03' on the other side. NDC 68180-568-01 - bottles of 100 Dispense in well-closed container with safety closure. Storage: Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Indications & Usage
INDICATIONS AND USAGE Hypertension Trandolapril tablets are indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive medication such as hydrochlorothiazide. Heart Failure Post Myocardial Infarction or Left-Ventricular Dysfunction Post Myocardial Infarction Trandolapril tablets are indicated in stable patients who have evidence of left-ventricular systolic dysfunction (identified by wall motion abnormalities) or who are symptomatic from congestive heart failure within the first few days after sustaining acute myocardial infarction. Administration of trandolapril to Caucasian patients has been shown to decrease the risk of death (principally cardiovascular death) and to decrease the risk of heart failure-related hospitalization (see CLINICAL PHARMACOLOGY -Heart Failure or Left-Ventricular Dysfunction Post Myocardial Infarction for details of the survival trial ).
Dosage and Administration
DOSAGE AND ADMINISTRATION Hypertension The recommended initial dosage of trandolapril tablets for patients not receiving a diuretic is 1 mg once daily in non-black patients and 2 mg in black patients. Dosage should be adjusted according to the blood pressure response. Generally, dosage adjustments should be made at intervals of at least 1 week. Most patients have required dosages of 2 to 4 mg once daily. There is little clinical experience with doses above 8 mg. Patients inadequately treated with once-daily dosing at 4 mg may be treated with twice-daily dosing. If blood pressure is not adequately controlled with trandolapril tablets monotherapy, a diuretic may be added. In patients who are currently being treated with a diuretic, symptomatic hypotension occasionally can occur following the initial dose of trandolapril tablets. To reduce the likelihood of hypotension, the diuretic should, if possible, be discontinued two to three days prior to beginning therapy with trandolapril tablets. (see WARNINGS ) . Then, if blood pressure is not controlled with trandolapril tablets alone, diuretic therapy should be resumed. If the diuretic cannot be discontinued, an initial dose of 0.5 mg trandolapril tablets should be used with careful medical supervision for several hours until blood pressure has stabilized. The dosage should subsequently be titrated (as described above) to the optimal response. (see WARNINGS , PRECAUTIONS , and DRUG INTERACTIONS .) Concomitant administration of trandolapril tablets with potassium supplements, potassium salt substitutes, or potassium sparing diuretics can lead to increases of serum potassium (see PRECAUTIONS .) Heart Failure Post Myocardial Infarction or Left-Ventricular Dysfunction Post Myocardial Infarction The recommended starting dose is 1 mg, once daily. Following the initial dose, all patients should be titrated (as tolerated) toward a target dose of 4 mg, once daily. If a 4 mg dose is not tolerated, patients can continue therapy with the greatest tolerated dose. Dosage Adjustment in Renal Impairment or Hepatic Cirrhosis For patients with a creatinine clearance <30 mL/min. or with hepatic cirrhosis, the recommended starting dose, based on clinical and pharmacokinetic data, is 0.5 mg daily. Patients should subsequently have their dosage titrated (as described above) to the optimal response.
