Drug Catalog - Product Detail
TROSPIUM CHLORIDE TAB 20 MG 60 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69097-0912-03 | CIPLA USA | 60 | 20MG | TABLET |
PACKAGE FILES

Generic Name
TROSPIUM CHLORIDE
Substance Name
TROSPIUM CHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA091688
Description
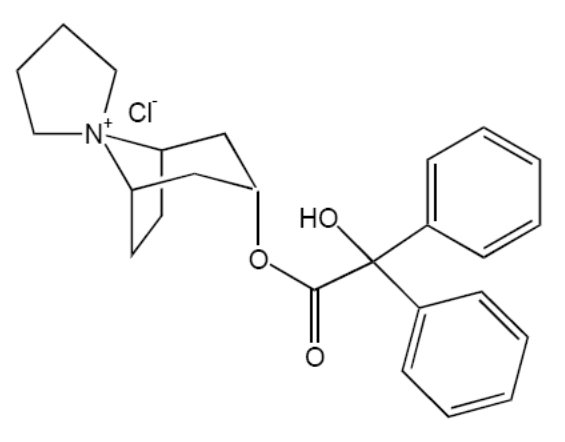
11 DESCRIPTION Trospium chloride, USP is a quaternary ammonium compound with the chemical name of Spiro[8azoniabicyclo[3.2.1]octane-8,1'-pyrrolidinium], 3-[(hydroxydiphenylacetyl)oxy]-, chloride, (1α, 3β, 5α). The empirical formula of trospium chloride, USP is C 25 H 30 ClNO 3 and its molecular weight is 427.97. The structural formula of trospium chloride is represented below: Trospium chloride, USP is a fine, colorless to slightly yellow, crystalline solid. The compound’s solubility in water is approximately 1 g per 2 mL. Each trospium chloride tablet contains 20 mg of trospium chloride, USP a muscarinic antagonist, for oral administration. Each tablet also contains the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, colloidal silicon dioxide, magnesium stearate, hypromellose 2910, titanium dioxide, polyethylene glycol 400, polysorbate 80, xylitol, sucralose, red iron oxide and yellow iron oxide. Meets USP Dissolution Test 2. trospium chloride structural formula
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Trospium chloride tablets, USP 20 mg (light yellow film coated, round biconvex tablets de-bossed with ‘IG’ on one side and ‘336’ on other) are supplied as follows: Bottles of 30s count (NDC 69097-912-02), Bottles of 60s count (NDC 69097-912-03) and Bottles of 1000s count (NDC 69097-912-15) Store at 20° to 25°C (68° to 77°F) (see USP Controlled Room Temperature).
Indications & Usage
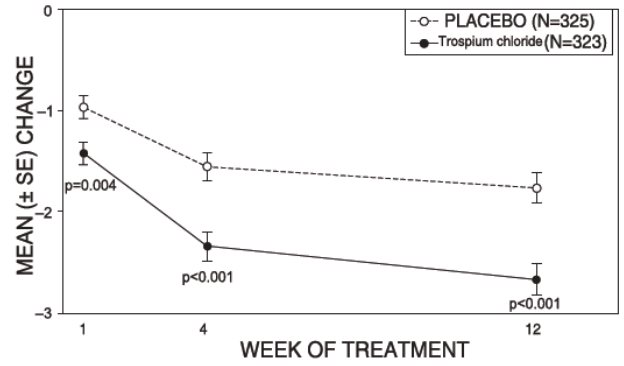
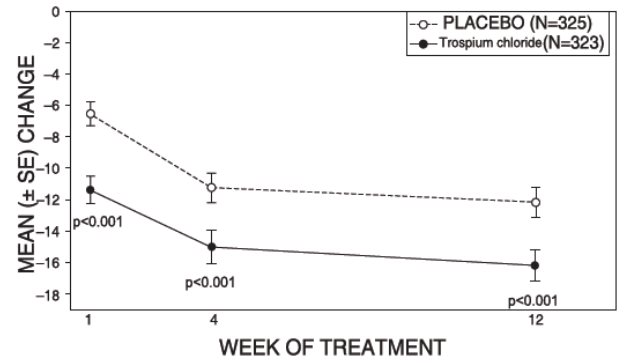
1 INDICATIONS AND USAGE Trospium chloride tablets are a muscarinic antagonist indicated for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency. Trospium chloride tablets are a muscarinic antagonist indicated for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION The recommended dose is 20 mg twice daily. Trospium chloride tablets should be dosed at least one hour before meals or given on an empty stomach. Dosage modification is recommended in the following patient populations: • For patients with severe renal impairment (creatinine clearance less than 30 mL/min), the recommended dose is 20 mg once daily at bedtime [ see Warnings and Precautions ( 5.5 ), Use in Specific Populations ( 8.6 ), and Clinical Pharmacology ( 12.3 ) ]. • In geriatric patients greater than or equal to 75 years of age, dose may be titrated down to 20 mg once daily based upon tolerability [ see Use in Specific Populations ( 8.5 ) ]. • The recommended dose of trospium chloride tablet is one 20 mg tablet twice daily. Trospium chloride tablets should be dosed with water on an empty stomach, at least one hour before a meal. ( 2 ) • For patients with severe renal impairment (creatinine clearance less than 30 mL/min), the recommended dose is 20 mg once daily at bedtime. ( 2 ) • In geriatric patients greater than or equal to 75 years of age, dose may be titrated down to 20 mg once daily based upon tolerability. ( 2 )
