Drug Catalog - Product Detail
VALACYCLOVIR HCL TB 1GM 250
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 59746-0325-37 | JUBILANT CADISTA | 250 | 1GM | TABLET |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
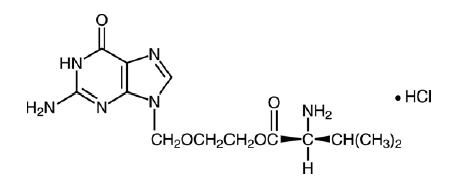
11 DESCRIPTION Valacyclovir hydrochloride,USP is the hydrochloride salt of the L -valyl ester of the antiviral drug acyclovir. Valacyclovir tablets, USP are for oral administration. Each tablet contains valacyclovir hydrochloride equivalent to 500 mg or 1 gram valacyclovir and the inactive ingredients crospovidone, FD&C Blue No. 2, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80 and titanium dioxide. The chemical name of valacyclovir hydrochloride is L -valine, 2-[(2-amino-1,6-dihydro-6-oxo-9 H -purin-9-yl)methoxy]ethyl ester, monohydrochloride. It has the following structural formula: Valacyclovir hydrochloride is a white to off-white powder with the molecular formula C 13 H 20 N 6 O 4 •HCl and a molecular weight of 360.80. The maximum solubility in water at 25°C is 174 mg/mL. The pK a s for valacyclovir hydrochloride are 1.90, 7.47, and 9.43. Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Valacyclovir tablets, USP 500 mg (blue colored, capsule shaped, film coated tablets debossed with "C324 500" on one side and plain on the other side) containing valacyclovir hydrochloride equivalent to 500 mg valacyclovir. Bottle of 10 (NDC 59746-324-24) Bottle of 30 (NDC 59746-324-30) Bottle of 90 (NDC 59746-324-90) Bottle of 100 (NDC 59746-324-01) Bottle of 250 (NDC 59746-324-37) Bottle of 300 (NDC 59746-324-47) Valacyclovir tablets, USP 1 gram (blue colored, capsule shaped, film coated tablets with partial score bar on both sides and debossed with "C325 1000" on one side and plain on the other) containing valacyclovir hydrochloride equivalent to 1 gram valacyclovir. Bottle of 10 (NDC 59746-325-24) Bottle of 30 (NDC 59746-325-30) Bottle of 90 (NDC 59746-325-90) Bottle of 100 (NDC 59746-325-01) Bottle of 250 (NDC 59746-325-37) Bottle of 300 (NDC 59746-325-47) Storage: Store at 20ºC-25°C (68ºF-77ºF), excursions permitted to 15°C-30°C (59ºF-86ºF). [See USP Controlled Room Temperature]. Dispense in a well-closed container as defined in the USP.
Indications & Usage
1 INDICATIONS AND USAGE Valacyclovir hydrochloride is a nucleoside analogue DNA polymerase inhibitor indicated for: Adult Patients ( 1.1 ) Cold Sores (Herpes Labialis) Genital Herpes Treatment in immunocompetent patients (initial or recurrent episode) Suppression in immunocompetent or HIV-infected patients Reduction of transmission Herpes Zoster Pediatric Patients ( 1.2 ) Cold Sores (Herpes Labialis) Chickenpox Limitations of Use ( 1.3 ) The efficacy and safety of valacyclovir hydrochloride have not been established in immunocompromised patients other than for the suppression of genital herpes in HIV-infected patients. 1.1 Adult Patients Cold Sores (Herpes Labialis): Valacyclovir Tablets, USP are indicated for treatment of cold sores (herpes labialis). The efficacy of valacyclovir hydrochloride initiated after the development of clinical signs of a cold sore (e.g., papule, vesicle, or ulcer) has not been established. Genital Herpes: Initial Episode: Valacyclovir Tablets, USP are indicated for treatment of the initial episode of genital herpes in immunocompetent adults. The efficacy of treatment with valacyclovir hydrochloride when initiated more than 72 hours after the onset of signs and symptoms has not been established. Recurrent Episodes: Valacyclovir Tablets, USP are indicated for treatment of recurrent episodes of genital herpes in immunocompetent adults. The efficacy of treatment with valacyclovir hydrochloride when initiated more than 24 hours after the onset of signs and symptoms has not been established. Suppressive Therapy: Valacyclovir Tablets, USP are indicated for chronic suppressive therapy of recurrent episodes of genital herpes in immunocompetent and in HIV‑infected adults. The efficacy and safety of valacyclovir hydrochloride for the suppression of genital herpes beyond 1 year in immunocompetent patients and beyond 6 months in HIV‑infected patients have not been established. Reduction of Transmission: Valacyclovir Tablets, USP are indicated for the reduction of transmission of genital herpes in immunocompetent adults. The efficacy of valacyclovir hydrochloride for the reduction of transmission of genital herpes beyond 8 months in discordant couples has not been established. The efficacy of valacyclovir hydrochloride for the reduction of transmission of genital herpes in individuals with multiple partners and non‑heterosexual couples has not been established. Safer sex practices should be used with suppressive therapy (see current Centers for Disease Control and Prevention [CDC] Sexually Transmitted Diseases Treatment Guidelines ). Herpes Zoster: Valacyclovir Tablets, USP are indicated for the treatment of herpes zoster (shingles) in immunocompetent adults. The efficacy of valacyclovir hydrochloride when initiated more than 72 hours after the onset of rash and the efficacy and safety of valacyclovir hydrochloride for treatment of disseminated herpes zoster have not been established. 1.2 Pediatric Patients Cold Sores (Herpes Labialis): Valacyclovir Tablets, USP are indicated for the treatment of cold sores (herpes labialis) in pediatric patients ≥12 years of age. The efficacy of valacyclovir hydrochloride initiated after the development of clinical signs of a cold sore (e.g., papule, vesicle, or ulcer) has not been established. Chickenpox: Valacyclovir Tablets, USP are indicated for the treatment of chickenpox in immunocompetent pediatric patients 2 to <18 years of age. Based on efficacy data from clinical studies with oral acyclovir, treatment with valacyclovir tablets, USP should be initiated within 24 hours after the onset of rash [see Clinical Studies ( 14.4 )] . 1.3 Limitations of Use The efficacy and safety of valacyclovir tablets, USP have not been established in: Immunocompromised patients other than for the suppression of genital herpes in HIV‑infected patients with a CD4+ cell count ≥100 cells/mm 3 . Patients <12 years of age with cold sores (herpes labialis). Patients <2 years of age or ≥18 years of age with chickenpox. Patients <18 years of age with genital herpes. Patients <18 years of age with herpes zoster. Neonates and infants as suppressive therapy following neonatal herpes simplex virus (HSV) infection.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Valacyclovir tablets, USP may be given without regard to meals. Valacyclovir oral suspension (25 mg/mLor 50 mg/mL) may be prepared extemporaneously from valacyclovir tablets, USP 500 mg for use in pediatric patients for whoma solid dosage form is not appropriate [see Dosage and Administration ( 2.3 )] . Adult Dosage ( 2.1 ) Cold Sores 2 grams every 12 hours for 1 day Genital Herpes Initial episode 1 gram twice daily for 10 days Recurrent episodes 500 mg twice daily for 3 days Suppressive therapy Immunocompetent patients 1 gram once daily Alternate dose in patients with ≤ 9 recurrences/yr 500 mg once daily HIV‑infected patients 500 mg twice daily Reduction of transmission 500 mg once daily Herpes Zoster 1 gram 3 times daily for 7 days Pediatric Dosage ( 2.2 ) Cold Sores (≥ 12 years of age) 2 grams every 12 hours for 1 day Chickenpox ( 2 to <18 years of age) 20 mg/kg 3 times daily for 5 days; not to exceed 1gram 3 times daily Valacyclovir oral suspension (25 mg/mL or 50 mg/mL) can be prepared from the valacyclovir tablets, USP 500 mg. ( 2.3 ) 2.1 Adult Dosing Recommendations Cold Sores (Herpes Labialis): The recommended dosage of valacyclovir hydrochloride for treatment of cold sores is 2 grams twice daily for 1 day taken 12 hours apart. Therapy should be initiated at the earliest symptom of a cold sore (e.g., tingling, itching, or burning). Genital Herpes: Initial Episode: The recommended dosage of valacyclovir hydrochloride for treatment of initial genital herpes is 1 gram twice daily for 10 days. Therapy was most effective when administered within 48 hours of the onset of signs and symptoms. Recurrent Episodes: The recommended dosage of valacyclovir hydrochloride for treatment of recurrent genital herpes is 500 mg twice daily for 3 days. Initiate treatment at the first sign or symptom of an episode. Suppressive Therapy: The recommended dosage of valacyclovir hydrochloride for chronic suppressive therapy of recurrent genital herpes is 1 gram once daily in patients with normal immune function. In patients with a history of 9 or fewer recurrences per year, an alternative dose is 500 mg once daily.In HIV‑infected patients with a CD4+ cell count > 100 cells/mm 3 , the recommended dosage of valacyclovir hydrochloride for chronic suppressive therapy of recurrent genital herpes is 500 mg twice daily. Reduction of Transmission: The recommended dosage of valacyclovir hydrochloride for reduction of transmission of genital herpes in patients with a history of 9 or fewer recurrences per year is 500 mg once daily for the source partner. Herpes Zoster: The recommended dosage of valacyclovir hydrochloride for treatment of herpes zoster is 1 gram 3 times daily for 7 days. Therapy should be initiated at the earliest sign or symptom of herpes zoster and is most effective when started within 48 hours of the onset of rash. 2.2 Pediatric Dosing Recommendations Cold Sores (Herpes Labialis): The recommended dosage of valacyclovir hydrochloride for the treatment of cold sores in pediatric patients ≥12 years of age is 2 grams twice daily for 1 day taken 12 hours apart. Therapy should be initiated at the earliest symptom of a cold sore (e.g., tingling, itching, or burning). Chickenpox: The recommended dosage of valacyclovir hydrochloride for treatment of chickenpox in immunocompetent pediatric patients 2 to <18 years of age is 20 mg/kg administered 3 times daily for 5 days. The total dose should not exceed 1 gram 3 times daily. Therapy should be initiated at the earliest sign or symptom [see Use in Specific Populations( 8.4 ),Clinical Pharmacology ( 12.3 ), Clinical Studies ( 14.4 )] . 2.3 Extemporaneous Preparation of Oral Suspension Ingredients and Preparation per USP-NF: valacyclovir hydrochloride 500 mg, cherry flavor, and Suspension Structured Vehicle USP-NF (SSV). Valacyclovir oral suspension (25 mg/mL or 50 mg/mL) should be prepared in lots of 100 mL. Prepare Suspension at Time of Dispensing as Follows: Prepare SSV accordingto the USP-NF. Using a pestle and mortar, grind the required number of valacyclovir tablets, USP 500 mg until a fine powder is produced (5 valacyclovir tablets, USP for 25 mg/mL suspension; 10 valacyclovir tablets, USP for 50 mg/mL suspension). Gradually add approximately 5 mL aliquots of SSV to the mortar and triturate the powder until a paste has been produced. Ensure that the powder has been adequately wetted. Continue to add approximately 5 mL aliquots of SSV to the mortar, mixing thoroughly between additions, until a concentrated suspension is produced, to a minimum total quantity of 20 mL SSV and a maximum total quantity of 40 mL SSV for both the 25 mg/mL and 50mg/mL suspensions. Transfer the mixture to a suitable 100 mL measuring flask. Transfer the cherry flavor* to the mortar and dissolve in approximately 5 mL of SSV. Once dissolved, add to the measuring flask. Rinse the mortar at least 3 times with approximately 5 mL aliquots of SSV, transferring the rinsing to the measuring flask between additions. Make the suspension to volume (100 mL) with SSV and shake thoroughly to mix. Transfer the suspension to an amber glass medicine bottle with a child-resistant closure. The prepared suspension should be labeled with the following information “Shake well before using. Store suspension between 2°C to 8°C (36°F to 46°F) in a refrigerator. Discard after 28 days.” *The amount of cherry flavor added is as instructed by the suppliers of the cherry flavor. 2.4 Patients With Renal Impairment Dosage recommendations for adult patients with reduced renal function are provided in Table 1 [see Use in Specific Populations ( 8.5 , 8.6 ), Clinical Pharmacology ( 12.3 )] . Data are not available for the use of valacyclovir hydrochloride in pediatric patients with a creatinine clearance <50 mL/min/1.73 m 2 . Table 1.Valacyclovir Hydrochloride Dosage Recommendations for Adults With Renal Impairment Indications Normal Dosage Regimen (Creatinine Clearance ≥ 50 mL/min) Creatinine Clearance (mL/min) 30-49 10-29 <10 Cold sores (Herpes labialis) Do not exceed 1 day of treatment. Two 2 gram doses taken 12 hours apart Two 1 gram doses taken 12 hours apart Two 500 mg doses taken 12 hours apart 500 mg single dose Genital herpes: Initial episode 1 gram every 12 hours no reduction 1 gram every 24 hours 500 mg every 24 hours Genital herpes: Recurrent episode 500 mg every 12 hours no reduction 500 mg every 24 hours 500 mg every 24 hours Genital herpes: Suppressive therapy Immunocompetent patients 1 gram every 24 hours no reduction 500 mg every 24 hours 500 mg every 24 hours Alternate dose for immunocompetent patients with ≤ 9 recurrences/year 500 mg every 24 hours no reduction 500 mg every 48 hours 500 mg every 48 hours HIV‑infected patients 500 mg every 12 hours no reduction 500 mg every 24 hours 500 mg every 24 hours Herpes zoster 1 gram every 8 hours 1 gram every 12 hours 1 gram every 24 hours 500 mg every 24 hours Hemodialysis: Patients requiring hemodialysis should receive the recommended dose of valacyclovir hydrochloride after hemodialysis. During hemodialysis, the half‑life of acyclovir after administration of valacyclovir hydrochloride is approximately 4 hours. About one third of acyclovir in the body is removed by dialysis during a 4‑hour hemodialysis session. Peritoneal Dialysis: There is no information specific to administration of valacyclovir hydrochloride in patients receiving peritoneal dialysis. The effect of chronic ambulatory peritoneal dialysis (CAPD) and continuous arteriovenous hemofiltration/dialysis (CAVHD) on acyclovir pharmacokinetics has been studied. The removal of acyclovir after CAPD and CAVHD is less pronounced than with hemodialysis, and the pharmacokinetic parameters closely resemble those observed in patients with end‑stage renal disease (ESRD) not receiving hemodialysis. Therefore, supplemental doses of valacyclovir hydrochloride should not be required following CAPD or CAVHD.
