Drug Catalog - Product Detail
VALSARTAN 160MG TB 90CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 59746-0362-90 | JUBILANT CADISTA | 90 | 160MG | TABLET |
PACKAGE FILES
Generic Name
VALSARTAN
Substance Name
VALSARTAN
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA203536
Description
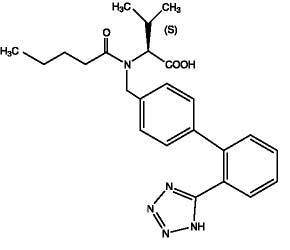
11 DESCRIPTION Valsartan USP is a nonpeptide, orally active, and specific angiotensin II receptor blocker acting on the AT 1 receptor subtype. Valsartan is chemically described as N -(1-oxopentyl)- N -[[2′-(1 H -tetrazol-5-yl) [1,1′-biphenyl]-4-yl]methyl]-L-valine. Its empirical formula is C 24 H 29 N 5 O 3 , its molecular weight is 435.52, and its structural formula is Valsartan USP is a white to off white hygroscopic powder. It is soluble in ethanol and methanol and slightly soluble in water. Valsartan is available as tablets for oral administration, containing 40 mg, 80 mg, 160 mg and 320 mg of valsartan USP. The inactive ingredients of the tablets are anhydrous lactose, crospovidone, dental-type silica, ferric oxide red (80 mg only), ferric oxide yellow, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, and titanium dioxide. FDA approved dissolution test specifications differ from USP. structuralformula
How Supplied
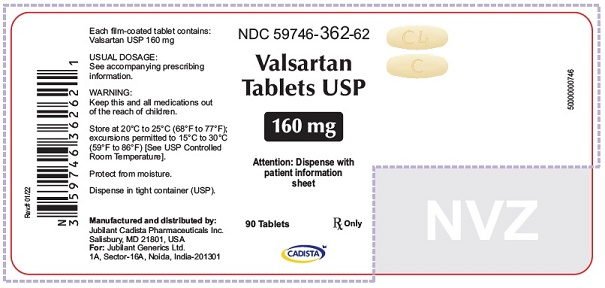
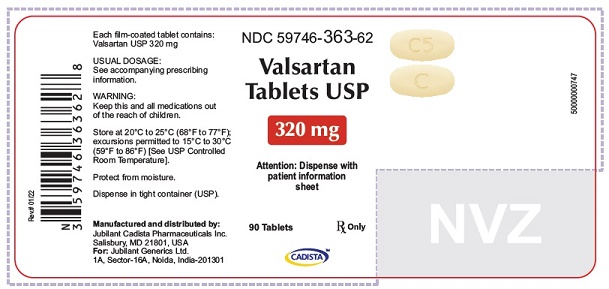
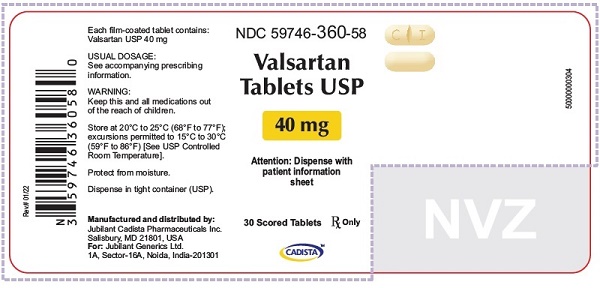
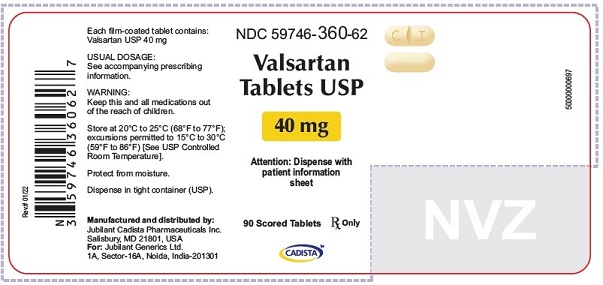
16 HOW SUPPLIED/STORAGE AND HANDLING Valsartan Tablets USP are available as follows: 40 mg tablets are yellow colored, capsule shaped, biconvex, film coated tablets, with a scoreline on one side, debossed with 'C' on one side of scoreline and 'I' on the other side of scoreline and plain on the other side. Bottle of 30’s (child-resistant closure) NDC 59746-360-58 Bottle of 90’s (child-resistant closure) NDC 59746-360-62 80 mg tablets are peach colored, round, biconvex, film coated tablets, debossed with 'C3' on one side and 'C' on other side. Bottle of 90’s (child-resistant closure) NDC 59746-361-62 160 mg tablets are yellow colored, oval shaped, biconvex, film coated tablets, debossed with 'C4' on one side and 'C' on other side. Bottle of 90’s (child-resistant closure) NDC 59746-362-62 320 mg tablets are yellow colored, oval shaped, biconvex, film coated tablets, debossed with 'C5' on one side and 'C' on other side. Bottle of 90’s (child-resistant closure) NDC 59746-363-62 Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F and 86°F) [See USP Controlled Room Temperature]. Protect from moisture. Dispense in tight container (USP).
Indications & Usage
1INDICATIONS AND USAGE Valsartan tablets are angiotensin II receptor blocker (ARB) indicated for: Hypertension, to lower blood pressure in adults and children 6 years and older. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions (1.1) Heart failure (NYHA class II-IV), to reduced hospitalization for heart failure in adults (1.2) Post-myocardial infarction, for the reduction of cardiovascular mortality in clinically stable patients with left ventricular failure or left ventricular dysfunction following myocardial infarction in adults( 1.3 ) 1.1 Hypertension Valsartan tablets are indicated for the treatment of hypertension, to lower blood pressure in adults and pediatric patients six years of age and older. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which valsartan principally belongs. There are no controlled trials in hypertensive patients demonstrating risk reduction with valsartan tablets. Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC). Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly. Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (e.g., patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal. Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy. Valsartan tablets may be used alone or in combination with other antihypertensive agents. Additional pediatric use information is approved for Novartis Pharmaceuticals Corporation's Diovan (valsartan) tablets. However, due to Novartis Pharmaceuticals Corporation's marketing exclusivity rights, this drug product is not labeled with that information. 1.2 Heart Failure Valsartan tablets are indicated to reduce the risk of hospitalization for heart failure in patients with heart failure (NYHA class II-IV). There is no evidence that valsartan tablets provides added benefits when it is used with an adequate dose of an ACE inhibitor [see Clinical Studies (14.2) ]. 1.3 Post-Myocardial Infarction In clinically stable patients with left ventricular failure or left ventricular dysfunction following myocardial infarction, Valsartan tablets is indicated to reduce the risk of cardiovascular mortality [see Clinical Studies (14.3) ].
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Indication Starting Dose Dose Range* Hypertension Adults (2.2 ) 80-160 mg once daily 80-320mg once daily 6-16 years ( 2. 3) 1.3 mg/kg once daily (up to 40 mg total) 1-4 mg/kg once daily Up to 160 mg daily Heart Failure ( 2.4) 40 mg twice daily 40-160 mg twice daily Post-Myocardial Infarction ( 2.5 ) 20 mg twice daily 20-160 mg twice daily 2.1 Important Dosage and Preparation Information Valsartan tablets and oral suspension are not substitutable on a milligram-per-milligram basis. Do not combine two dosage forms to achieve the total dose. The systemic exposure to valsartan (AUC) is 60% higher with the suspension compared to tablets [see Clinical Pharmacology (12.3)]. Use of the oral suspension is recommended: in patients ≥ 6 years of age who cannot swallow tablets and in pediatric patients for whom the calculated dose (mg/kg) does not correspond to the available tablet strengths of valsartan tablet. When switching between suspension and tablets, the dose of valsartan may need to be adjusted. Preparation of Suspension (for 160 mL of a 4 mg/mL suspension) Add 80 mL of Ora-Plus ®* oral suspending vehicle to an amber glass bottle containing 8 valsartan 80 mg tablets and shake for a minimum of 2 minutes. Allow the suspension to stand for a minimum of 1 hour. After the standing time, shake the suspension for a minimum of 1 additional minute. Add 80 mL of Ora-Sweet SF ®* oral sweetening vehicle to the bottle and shake the suspension for at least 10 seconds to disperse the ingredients. The suspension is homogenous and can be stored for either up to 30 days at room temperature (below 30°C/86°F) or up to 75 days at refrigerated conditions (2°C to 8°C/35°F to 46°F) in the glass bottle with a child-resistant screw-cap closure. Shake the bottle well (at least 10 seconds) prior to dispensing the suspension. *Ora-Sweet SF ® and Ora-Plus ® are registered trademarks of Paddock Laboratories, Inc. Additional pediatric use information is approved for Novartis Pharmaceuticals Corporation's Diovan (valsartan) tablets. However, due to Novartis Pharmaceuticals Corporation's marketing exclusivity rights, this drug product is not labeled with that information. 2.2 Adult Hypertension The recommended starting dose of valsartan tablets is 80 mg or 160 mg once daily when used as monotherapy in patients who are not volume-depleted. Patients requiring greater reductions may be started at the higher dose. Valsartan tablets may be used over a dose range of 80 mg to 320 mg daily, administered once a day. The antihypertensive effect is substantially present within 2 weeks and maximal reduction is generally attained after 4 weeks. If additional antihypertensive effect is required over the starting dose range, the dose may be increased to a maximum of 320 mg or a diuretic may be added. Addition of a diuretic has a greater effect than dose increases beyond 80 mg. Valsartan tablets may be administered with other antihypertensive agents. 2.3 Pediatric Hypertension 6 to 16 Years of Age The usual recommended starting dose is 1 mg/kg once daily (up to 40 mg total). A higher starting dose of 2 mg/kg may be considered in selected cases when a greater reduction of blood pressure is needed. The dosage should be adjusted according to blood pressure response and tolerability, up to a maximum dose of 4 mg/kg once daily (maximum daily dose 160 mg). No data are available in pediatric patients either undergoing dialysis or with a glomerular filtration rate < 30 mL/min/1.73 m2 [see Use in Specific Populations (8.4)]. Use of valsartan tablets is not recommended in children less than 1 year of age [see Adverse Reactions (6.1) , Pediatric Use in Specific Populations (8.4), Nonclinical Toxicology (13.2)]. Additional pediatric use information is approved for Novartis Pharmaceuticals Corporation's Diovan (valsartan) tablets. However, due to Novartis Pharmaceuticals Corporation's marketing exclusivity rights, this drug product is not labeled with that information. 2.4 Heart Failure The recommended starting dose of valsartan is 40 mg twice daily. Uptitrate to 80 mg and 160 mg twice daily or to the highest dose tolerated by the patient. Consider reducing the dose of concomitant diuretics. The maximum daily dose administered in clinical trials is 320 mg in divided doses. 2.5 Post-Myocardial Infarction Valsartan tablets may be initiated as early as 12 hours after a myocardial infarction. The recommended starting dose of valsartan tablets is 20 mg twice daily. Patients may be uptitrated within 7 days to 40 mg twice daily, with subsequent titrations to a target maintenance dose of 160 mg twice daily, as tolerated by the patient. If symptomatic hypotension or renal dysfunction occurs, consider dosage reduction. Valsartan tablets may be given with other standard post-myocardial infarction treatment, including thrombolytics, aspirin, beta-blockers, and statins. 2.6 Missed Dose If a dose of valsartan tablet is missed, it should be administered as soon as possible, unless it is almost time for the next dose. The dose should not be doubled to make up for a missed dose.
