Drug Catalog - Product Detail
VANCOMYCIN HCL USP CP 250MG 2X10 UD
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 62559-0391-20 | ANI PHARMACEUTICALS | 20 | 250MG | CAPSULE |
PACKAGE FILES

Generic Name
VANCOMYCIN HYDROCHLORIDE
Substance Name
VANCOMYCIN HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
NDA050606
Description
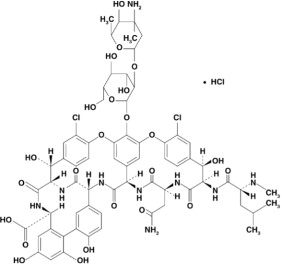
11 DESCRIPTION Vancomycin Hydrochloride Capsules USP for oral administration contain chromatographically purified vancomycin hydrochloride, a tricyclic glycopeptide antibiotic derived from Amycolatopsis orientalis (formerly Nocardia orientalis ), which has the chemical formula C 66 H 75 Cl 2 N 9 O 24 •HCl. The molecular weight of vancomycin hydrochloride is 1485.73; 500 mg of the base is equivalent to 0.34 mmol. Each capsule contains 125 mg vancomycin (equivalent to 128 mg vancomycin hydrochloride) or 250 mg vancomycin (equivalent to 256 mg vancomycin hydrochloride). The capsules also contain FD&C Blue No. 2, gelatin, iron oxide, polyethylene glycol, titanium dioxide, and other inactive ingredients. Vancomycin hydrochloride has the structural formula: chemical structure
How Supplied
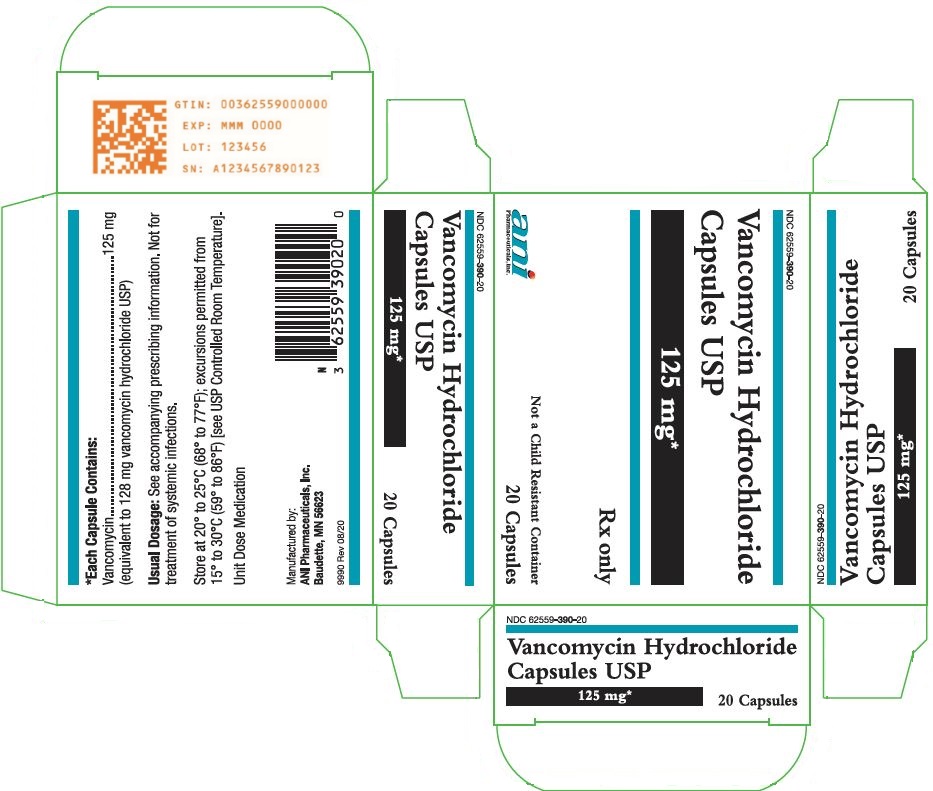
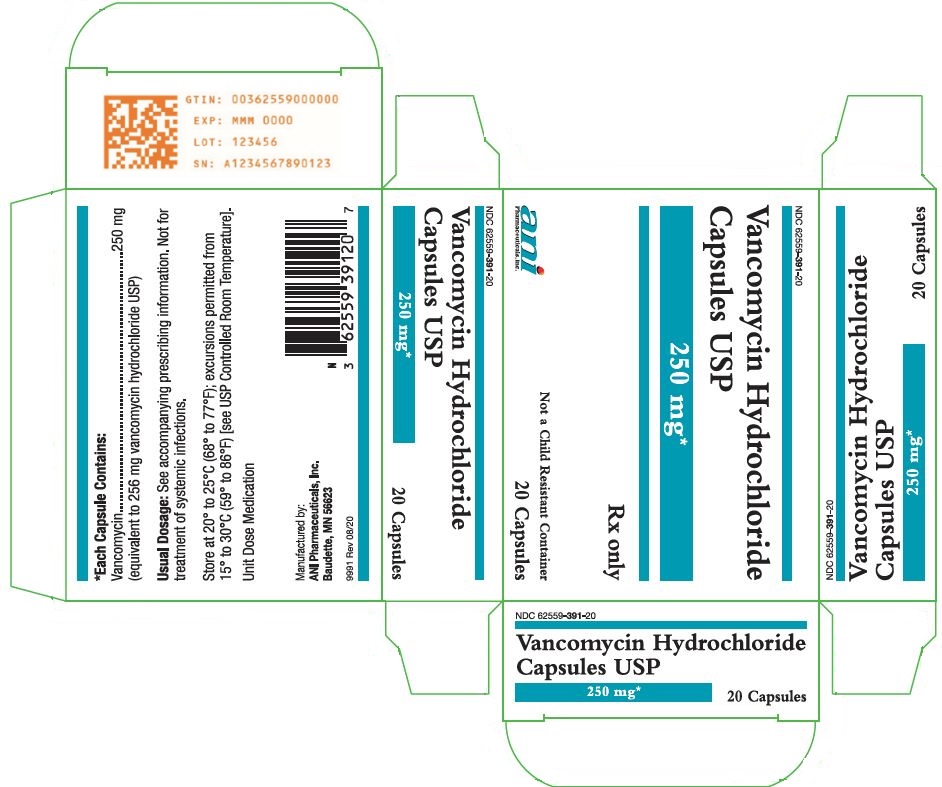
16 HOW SUPPLIED/STORAGE AND HANDLING Vancomycin Hydrochloride Capsules USP are available in: The 125 mg (equivalent to vancomycin) capsules have an opaque blue cap and opaque brown body imprinted with “3125” on the cap and “VANCOCIN HCL 125 MG” on the body in white ink. NDC 62559-390-20: Carton containing 2 blister packs. Each blister pack contains 10 capsules for a total of 20 capsules per carton. NDC 62559-390-50: Bottle of 50 capsules. The 250 mg (equivalent to vancomycin) capsules have an opaque blue cap and opaque lavender body imprinted with “3126” on the cap and “VANCOCIN HCL 250 MG” on the body in white ink. NDC 62559-391-20: Carton containing 2 blister packs. Each blister pack contains 10 capsules for a total of 20 capsules per carton. NDC 62559-391-50: Bottle of 50 capsules. Store at 20° to 25°C (68° to 77°F); excursions permitted from 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Vancomycin Hydrochloride Capsules are indicated for the treatment of Clostridioides difficile -associated diarrhea. Vancomycin Hydrochloride Capsules are also used for the treatment of enterocolitis caused by Staphylococcus aureus (including methicillin-resistant strains) in adult and pediatric patients less than 18 years of age. Limitations of Use • Parenteral administration of vancomycin is not effective for the above infections; therefore, Vancomycin Hydrochloride Capsules must be given orally for these infections. • Orally administered Vancomycin Hydrochloride Capsules are not effective for other types of infections. To reduce the development of drug-resistant bacteria and maintain the effectiveness of Vancomycin Hydrochloride Capsules and other antibacterial drugs, Vancomycin Hydrochloride Capsules should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. Vancomycin Hydrochloride Capsules are a glycopeptide antibacterial indicated in adult and pediatric patients (less than 18 years of age) for the treatment of: ( 1 ) • Clostridioides difficile-associated diarrhea • Enterocolitis caused by Staphylococcus aureus (including methicillin-resistant strains) Limitations of Use: ( 1 ) ( 5.1 ) • Parenteral administration of vancomycin is not effective for the above infections; therefore, Vancomycin Hydrochloride Capsules must be given orally for these infections. • Orally administered Vancomycin Hydrochloride Capsules are not effective for other types of infections. To reduce the development of drug-resistant bacteria and maintain the effectiveness of Vancomycin Hydrochloride Capsules and other antibacterial drugs, Vancomycin Hydrochloride Capsules should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION • C. difficile- associated diarrhea: • Adult Patients (18 years of age or greater): 125 mg orally 4 times daily for 10 days. ( 2.1 ) • Pediatric Patients (less than 18 years of age): 40 mg/kg in 3 or 4 divided doses for 7 to 10 days. The total daily dosage should not exceed 2 g. ( 2.2 ) • Staphylococcal enterocolitis: • Adult Patients (18 years of age or greater): 500 mg to 2 g orally in 3 or 4 divided doses for 7 to 10 days. ( 2.1 ) • Pediatric Patients (less than 18 years of age): 40 mg/kg in 3 or 4 divided doses for 7 to 10 days. The total daily dosage should not exceed 2 g. ( 2.2 ) 2.1 Adults Vancomycin Hydrochloride Capsules are used in treating C. difficile -associated diarrhea and staphylococcal enterocolitis. • C. difficile- associated diarrhea: The recommended dose is 125 mg administered orally 4 times daily for 10 days. • Staphylococcal enterocolitis: Total daily dosage is 500 mg to 2 g administered orally in 3 or 4 divided doses for 7 to 10 days. 2.2 Pediatric Patients (less than 18 years of age) For both C. difficile -associated diarrhea and staphylococcal enterocolitis, the usual daily dosage is 40 mg/kg in 3 or 4 divided doses for 7 to 10 days. The total daily dosage should not exceed 2 g.
