Drug Catalog - Product Detail
VASCEPA CAP 1GM 120CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 52937-0001-20 | AMARIN PHARMA | 120 | 1GM | CAPSULE |
PACKAGE FILES

Generic Name
ICOSAPENT ETHYL
Substance Name
ICOSAPENT ETHYL
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
NDA202057
Description
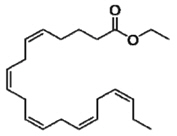
11 DESCRIPTION VASCEPA, a lipid-regulating agent, is supplied as either a 0.5 gram or a 1 gram amber-colored, liquid-filled soft gelatin capsule for oral use. Each VASCEPA capsule contains either 0.5 grams of icosapent ethyl (in a 0.5 gram capsule) or 1 gram of icosapent ethyl (in a 1 gram capsule). Icosapent ethyl is an ethyl ester of the omega-3 fatty acid eicosapentaenoic acid (EPA). The empirical formula of icosapent ethyl is C 22 H 34 O 2 and the molecular weight is 330.51. The chemical name for icosapent ethyl is ethyl all-cis-5,8,11,14,17-icosapentaenoate with the following chemical structure: VASCEPA capsules also contain the following inactive ingredients: tocopherol, gelatin, glycerin, maltitol, sorbitol, and purified water. Chemical Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING VASCEPA (icosapent ethyl) capsules are supplied as Strength Quantity Description NDC 0.5 gram capsules Bottles of 240 amber-colored soft-gelatin capsules imprinted with V500 52937-003-40 1 gram capsules Bottles of 120 amber-colored soft-gelatin capsules imprinted with VASCEPA 52937-001-20 Store at 20° to 25° C (68° to 77°F); excursions permitted to 15° to 30° C (59° to 86°F) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE VASCEPA ® (icosapent ethyl) is indicated: as an adjunct to maximally tolerated statin therapy to reduce the risk of myocardial infarction, stroke, coronary revascularization, and unstable angina requiring hospitalization in adult patients with elevated triglyceride (TG) levels (≥ 150 mg/dL) and established cardiovascular disease or diabetes mellitus and 2 or more additional risk factors for cardiovascular disease. as an adjunct to diet to reduce TG levels in adult patients with severe (≥ 500 mg/dL) hypertriglyceridemia. Limitations of Use: The effect of VASCEPA on the risk for pancreatitis in patients with severe hypertriglyceridemia has not been determined. VASCEPA is an ethyl ester of eicosapentaenoic acid (EPA) indicated: as an adjunct to maximally tolerated statin therapy to reduce the risk of myocardial infarction, stroke, coronary revascularization, and unstable angina requiring hospitalization in adult patients with elevated triglyceride (TG) levels(≥ 150 mg/dL) and established cardiovascular disease or diabetes mellitus and 2 or more additional risk factors for cardiovascular disease. ( 1 ) as an adjunct to diet to reduce TG levels in adult patients with severe (≥ 500 mg/dL) hypertriglyceridemia. ( 1 ) Limitations of Use: The effect of VASCEPA on the risk for pancreatitis in patients with severe hypertriglyceridemia has not been determined. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Assess lipid levels before initiating therapy. Identify other causes of high triglyceride levels and manage as appropriate. ( 2.1 ) Patients should engage in appropriate nutritional intake and physical activity before receiving VASCEPA, which should continue during treatment. ( 2.1 ) The daily dose of VASCEPA is 4 grams per day taken as either four 0.5 gram capsules twice daily with food or two 1 gram capsules twice daily with food. ( 2.2 ) Advise patients to swallow capsules whole. Do not break open, crush, dissolve, or chew VASCEPA. ( 2.2 ) 2.1 Prior to Initiation of VASCEPA Assess lipid levels before initiating therapy. Identify other causes (e.g., diabetes mellitus, hypothyroidism, or medications) of high triglyceride levels and manage as appropriate. Patients should engage in appropriate nutritional intake and physical activity before receiving VASCEPA, which should continue during treatment with VASCEPA. 2.2 Dosage and Administration The daily dose of VASCEPA is 4 grams per day taken as either: four 0.5 gram capsules twice daily with food; or as two 1 gram capsules twice daily with food. Advise patients to swallow VASCEPA capsules whole. Do not break open, crush, dissolve, or chew VASCEPA.
