Drug Catalog - Product Detail
VERAPAMIL HCL ER TB 240MG 500
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68462-0260-05 | GLENMARK PHARMACEUTICALS | 500 | 240MG | TABLET |
PACKAGE FILES


Generic Name
Substance Name
Product Type
Route
Application Number
Description
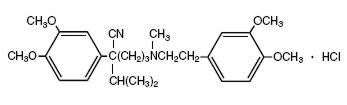
DESCRIPTION Verapamil hydrochloride extended-release tablets USP are calcium ion influx inhibitor (slow channel blocker or calcium ion antagonist). Verapamil hydrochloride extended-release tablets USP are available for oral administration as brown colored, oval, biconvex, film-coated tablets containing 120 mg verapamil hydrochloride USP, as brown colored, oval, biconvex, film-coated tablets containing 180 mg verapamil hydrochloride USP, and as brown colored, oval, biconvex, film-coated tablets containing 240 mg verapamil hydrochloride USP. The tablets are designed for sustained release of the drug in the gastrointestinal tract; sustained-release characteristics are not altered when the tablet is divided in half. The structural formula of verapamil HCl USP is given below: C 27 H 38 N 2 O 4 ·HCl..........M.W. 491.06 Benzeneacetonitrile, α[3-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]propyl]-3,4- dimethoxy-α-(1-methylethyl) hydrochloride Verapamil HCl USP is an almost white, crystalline powder, practically free of odor, with a bitter taste. It is soluble in water, chloroform and methanol. Verapamil HCl USP is not chemically related to other cardioactive drugs. In addition to verapamil HCl USP, the verapamil hydrochloride extended-release tablets USP contain the following ingredients: colloidal silicon dioxide, sodium alginate, hypromellose, magnesium stearate, microcrystalline cellulose, povidone, polyethylene glycol and titanium dioxide. The following are the color additives per tablet strength: Strength (mg) Color Additive(s) 120 Ferric Oxide Yellow, Ferric Oxide Red and Ferric Oxide Black 180 Ferric Oxide Yellow, Ferric Oxide Red and Ferric Oxide Black 240 Ferric Oxide Yellow, Ferric Oxide Red and Ferric Oxide Black Verapamil hydrochloride extended-release tablets USP, 120 mg, 180 mg and 240 mg meet USP Dissolution Test 1. Structure of Verapamil Hydrochloride
How Supplied
HOW SUPPLIED Verapamil hydrochloride extended-release tablets USP, 120 mg are supplied as brown colored, oval, biconvex, film-coated tablets with ‘292’ debossed on one side and plain on the other side. 120 mg (brown colored) Bottles of 90 - NDC 68462-292-90 Bottles of 100 - NDC 68462-292-01 Bottles of 500 - NDC 68462-292-05 Verapamil hydrochloride extended-release tablets USP, 180 mg are supplied as brown colored, oval, biconvex, film-coated tablets with ‘293’ debossed on one side and breakline on the other side. 180 mg (brown colored) Bottles of 90 - NDC 68462-293-90 Bottles of 100 - NDC 68462-293-01 Bottles of 500 - NDC 68462-293-05 Verapamil hydrochloride extended-release tablets USP, 240 mg are supplied as brown colored, oval, biconvex, film-coated tablets with ‘G74’ debossed on one side and breakline on the other side. 240 mg (brown colored) Bottles of 30 - NDC 68462-260-30 Bottles of 90 - NDC 68462-260-90 Bottles of 100 - NDC 68462-260-01 Bottles of 500 - NDC 68462-260-05 Bottles of 1000 - NDC 68462-260-10 Storage: Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from light and moisture. Dispense in a tight, light-resistant container as defined in the USP. You may report side effects to FDA at 1-800-FDA-1088 or Glenmark Pharmaceuticals Inc., USA at 1 (888)721-7115.
Indications & Usage
INDICATIONS AND USAGE Verapamil hydrochloride extended-release tablets USP are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including this drug. Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC). Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly. Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal. Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Dosage and Administration
DOSAGE AND ADMINISTRATION Essential hypertension The dose of verapamil hydrochloride extended-release tablets USP should be individualized by titration and the drug should be administered with food. Initiate therapy with 180 mg of sustained-release verapamil HCl, verapamil hydrochloride extended-release tablets, given in the morning. Lower initial doses of 120 mg a day may be warranted in patients who may have an increased response to verapamil (e.g., the elderly or small people). Upward titration should be based on therapeutic efficacy and safety evaluated weekly and approximately 24 hours after the previous dose. The antihypertensive effects of verapamil hydrochloride extended-release tablets are evident within the first week of therapy. If adequate response is not obtained with 180 mg of verapamil hydrochloride extended-release tablets, the dose may be titrated upward in the following manner: 1. 240 mg each morning, 2. 180 mg each morning plus 180 mg each evening; or 240 mg each morning plus 120 mg each evening, 3. 240 mg every twelve hours. When switching from immediate-release verapamil hydrochloride tablets to verapamil hydrochloride extended-release tablets, the total daily dose in milligrams may remain the same.
