Drug Catalog - Product Detail
XARELTO TAB 20MG 90CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 50458-0579-90 | JANSSEN | 90 | 20MG | TABLET |
PACKAGE FILES
















Generic Name
RIVAROXABAN
Substance Name
RIVAROXABAN
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
NDA215859
Description
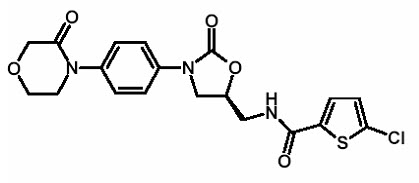
11 DESCRIPTION Rivaroxaban, a factor Xa (FXa) inhibitor, is the active ingredient in XARELTO ® Tablets and XARELTO ® for oral suspension with the chemical name 5-Chloro-N-({(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}methyl)-2-thiophenecarboxamide. The molecular formula of rivaroxaban is C 19 H 18 ClN 3 O 5 S and the molecular weight is 435.89. The structural formula is: Rivaroxaban is a pure ( S )-enantiomer. It is an odorless, non-hygroscopic, white to yellowish powder. Rivaroxaban is only slightly soluble in organic solvents (e.g., acetone, polyethylene glycol 400) and is practically insoluble in water and aqueous media. Each XARELTO tablet contains 2.5 mg, 10 mg, 15 mg, or 20 mg of rivaroxaban. The inactive ingredients of XARELTO are: croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. Additionally, the proprietary film coating mixture used for XARELTO 2.5 mg is Opadry ® Light Yellow, containing ferric oxide yellow, hypromellose, polyethylene glycol 3350, and titanium dioxide, and for XARELTO 10 mg tablets is Opadry ® Pink and for XARELTO 15 mg tablets is Opadry ® Red, both containing ferric oxide red, hypromellose, polyethylene glycol 3350, and titanium dioxide, and for XARELTO 20 mg tablets is Opadry ® II Dark Red, containing ferric oxide red, polyethylene glycol 3350, polyvinyl alcohol (partially hydrolyzed), talc, and titanium dioxide. XARELTO for oral suspension is supplied as granules in bottles containing 155 mg of rivaroxaban (1 mg of rivaroxaban per mL after reconstitution). The inactive ingredients are: anhydrous citric acid, hypromellose, mannitol, microcrystalline cellulose and carboxymethylcellulose sodium, sodium benzoate, sucralose, sweet and creamy flavor and xanthan gum. Chemical Structure
How Supplied
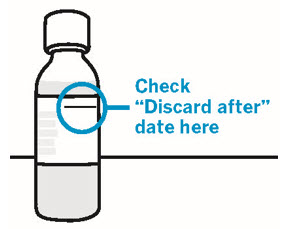
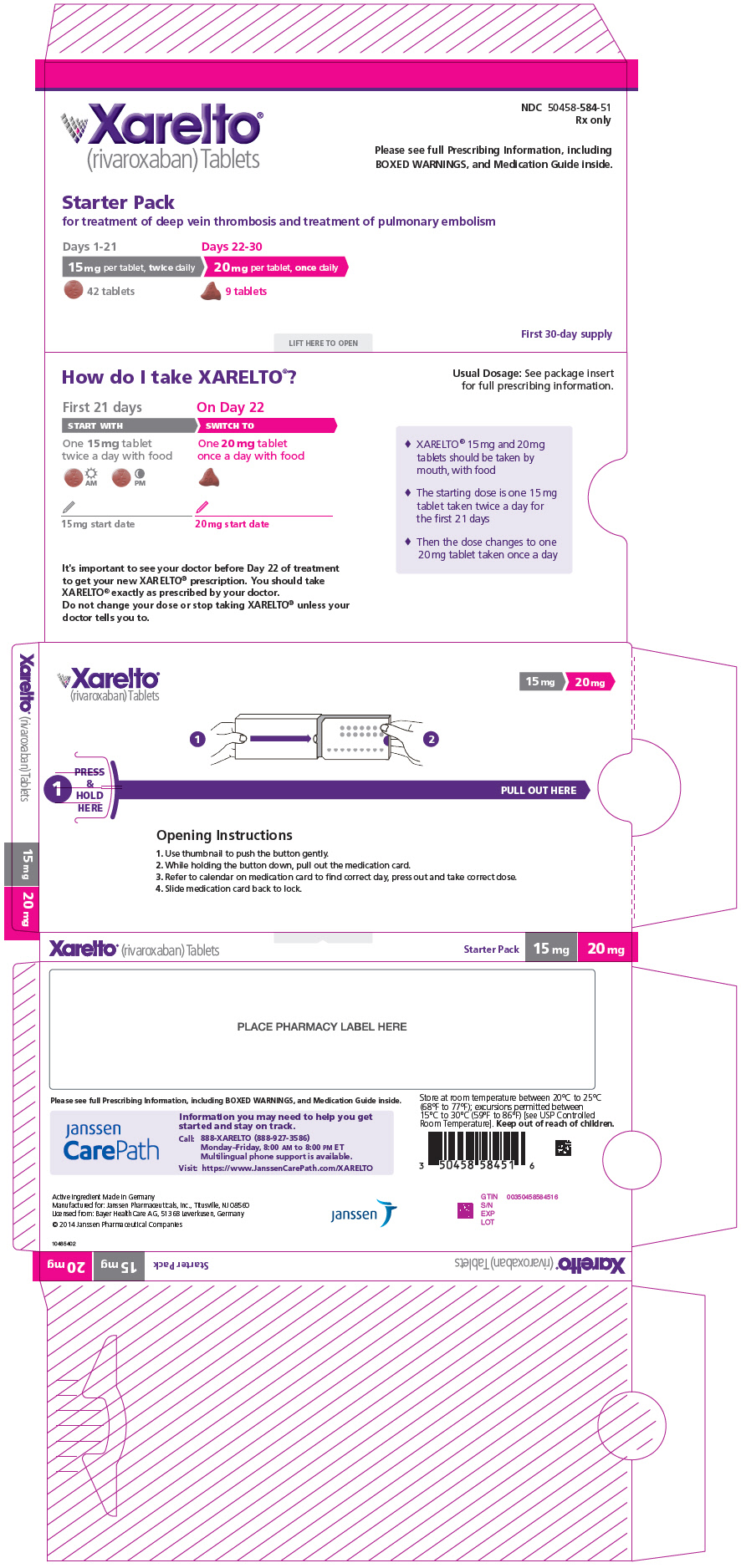
16 HOW SUPPLIED/STORAGE AND HANDLING XARELTO ® (rivaroxaban) Tablets are available in the strengths and packages listed below: 2.5 mg tablets are round, light yellow, and film-coated with a triangle pointing down above a "2.5" marked on one side and "Xa" on the other side. The tablets are supplied in the packages listed: NDC 50458-577-60 Bottle containing 60 tablets NDC 50458-577-18 Bottle containing 180 tablets NDC 50458-577-10 Blister package containing 100 tablets (10 blister cards containing 10 tablets each) 10 mg tablets are round, light red, biconvex film-coated tablets marked with a triangle pointing down above a "10" on one side, and "Xa" on the other side. The tablets are supplied in the packages listed: NDC 50458-580-30 Bottle containing 30 tablets NDC 50458-580-90 Bottle containing 90 tablets NDC 50458-580-10 Blister package containing 100 tablets (10 blister cards containing 10 tablets each) 15 mg tablets are round, red, biconvex film-coated tablets with a triangle pointing down above a "15" marked on one side and "Xa" on the other side. The tablets are supplied in the packages listed: NDC 50458-578-30 Bottle containing 30 tablets NDC 50458-578-90 Bottle containing 90 tablets NDC 50458-578-10 Blister package containing 100 tablets (10 blister cards containing 10 tablets each) 20 mg tablets are triangle-shaped, dark red film-coated tablets with a triangle pointing down above a "20" marked on one side and "Xa" on the other side. The tablets are supplied in the packages listed: NDC 50458-579-30 Bottle containing 30 tablets NDC 50458-579-90 Bottle containing 90 tablets NDC 50458-579-89 Bulk bottle containing 1000 tablets NDC 50458-579-10 Blister package containing 100 tablets (10 blister cards containing 10 tablets each) Starter Pack for treatment of deep vein thrombosis and treatment of pulmonary embolism: NDC 50458-584-51 30-day starter blister pack containing 51 tablets: 42 tablets of 15 mg and 9 tablets of 20 mg XARELTO ® (rivaroxaban) for oral suspension is available in the strength and package listed below: NDC 50458-575-01 Supplied as white to off-white granules in an amber glass bottle containing 155 mg rivaroxaban packaged with two oral dosing syringes. After reconstitution with 150 mL of purified water, 1 mL of the suspension contains 1 mg rivaroxaban. Discard reconstituted suspension after "Discard after" date written on the bottle. Storage of tablets, granules and reconstituted suspension: Store at room temperature between 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Do not freeze the granules or reconstituted suspension. Keep out of the reach of children.
Indications & Usage
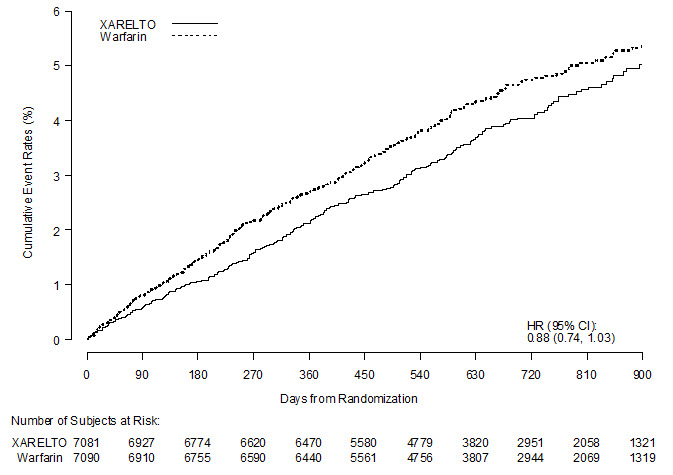
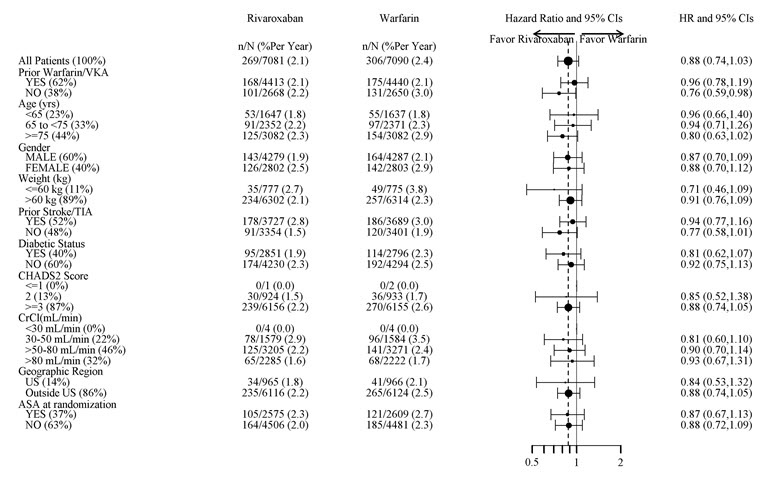
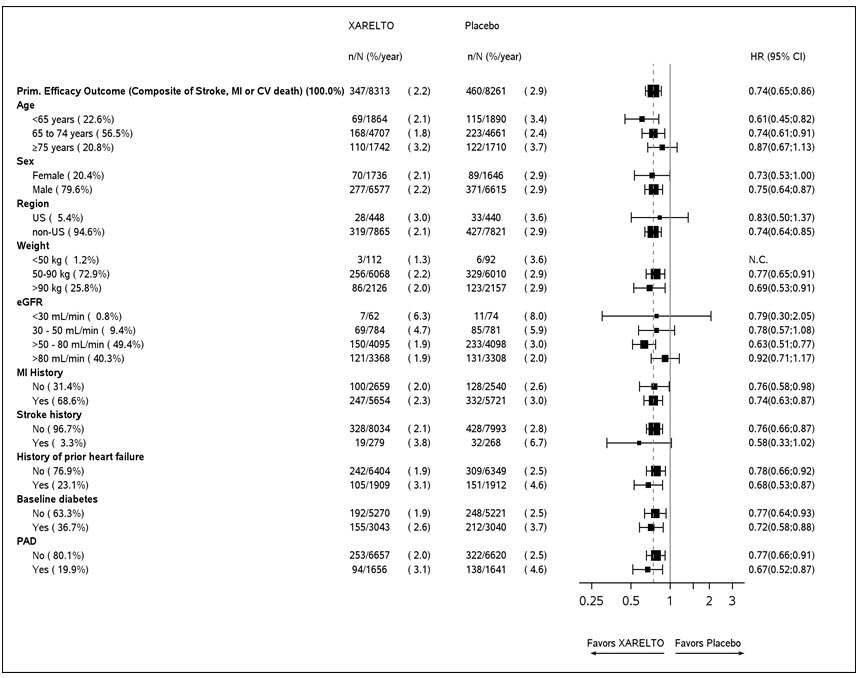
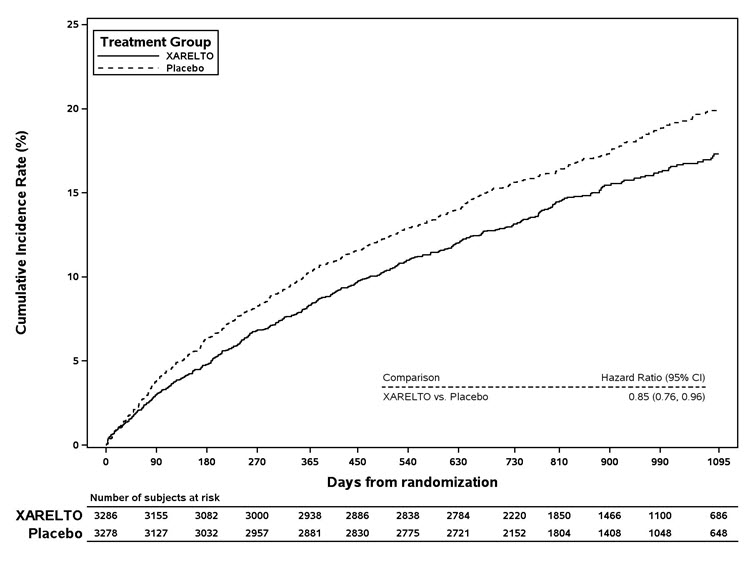
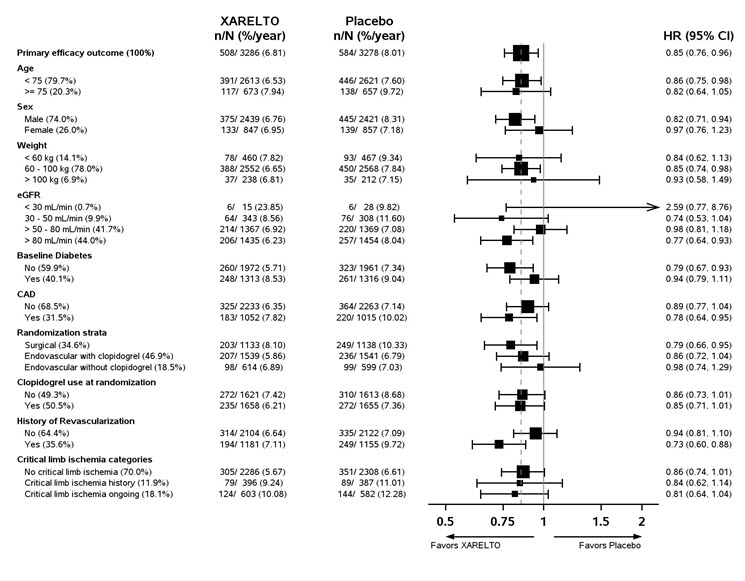
1 INDICATIONS AND USAGE XARELTO is a factor Xa inhibitor indicated: to reduce risk of stroke and systemic embolism in nonvalvular atrial fibrillation ( 1.1 ) for treatment of deep vein thrombosis (DVT) ( 1.2 ) for treatment of pulmonary embolism (PE) ( 1.3 ) for reduction in the risk of recurrence of DVT or PE ( 1.4 ) for the prophylaxis of DVT, which may lead to PE in patients undergoing knee or hip replacement surgery ( 1.5 ) for prophylaxis of venous thromboembolism (VTE) in acutely ill medical patients ( 1.6 ) to reduce the risk of major cardiovascular events in patients with coronary artery disease (CAD) ( 1.7 ) to reduce the risk of major thrombotic vascular events in patients with peripheral artery disease (PAD), including patients after recent lower extremity revascularization due to symptomatic PAD ( 1.8 ) for treatment of VTE and reduction in the risk of recurrent VTE in pediatric patients from birth to less than 18 years ( 1.9 ) for thromboprophylaxis in pediatric patients 2 years and older with congenital heart disease after the Fontan procedure ( 1.10 ) 1.1 Reduction of Risk of Stroke and Systemic Embolism in Nonvalvular Atrial Fibrillation XARELTO is indicated to reduce the risk of stroke and systemic embolism in adult patients with nonvalvular atrial fibrillation. There are limited data on the relative effectiveness of XARELTO and warfarin in reducing the risk of stroke and systemic embolism when warfarin therapy is well-controlled [see Clinical Studies (14.1) ]. 1.2 Treatment of Deep Vein Thrombosis XARELTO is indicated for the treatment of deep vein thrombosis (DVT). 1.3 Treatment of Pulmonary Embolism XARELTO is indicated for the treatment of pulmonary embolism (PE). 1.4 Reduction in the Risk of Recurrence of Deep Vein Thrombosis and/or Pulmonary Embolism XARELTO is indicated for the reduction in the risk of recurrence of DVT and/or PE in adult patients at continued risk for recurrent DVT and/or PE after completion of initial treatment lasting at least 6 months. 1.5 Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery XARELTO is indicated for the prophylaxis of DVT, which may lead to PE in adult patients undergoing knee or hip replacement surgery. 1.6 Prophylaxis of Venous Thromboembolism in Acutely Ill Medical Patients at Risk for Thromboembolic Complications Not at High Risk of Bleeding XARELTO is indicated for the prophylaxis of venous thromboembolism (VTE) and VTE related death during hospitalization and post hospital discharge in adult patients admitted for an acute medical illness who are at risk for thromboembolic complications due to moderate or severe restricted mobility and other risk factors for VTE and not at high risk of bleeding [see Warnings and Precautions (5.2) and Clinical Studies (14.5) ] . 1.7 Reduction of Risk of Major Cardiovascular Events in Patients with Coronary Artery Disease (CAD) XARELTO, in combination with aspirin, is indicated to reduce the risk of major cardiovascular events (cardiovascular death, myocardial infarction, and stroke) in adult patients with coronary artery disease. 1.8 Reduction of Risk of Major Thrombotic Vascular Events in Patients with Peripheral Artery Disease (PAD), Including Patients after Lower Extremity Revascularization due to Symptomatic PAD XARELTO, in combination with aspirin, is indicated to reduce the risk of major thrombotic vascular events (myocardial infarction, ischemic stroke, acute limb ischemia, and major amputation of a vascular etiology) in adult patients with PAD, including patients who have recently undergone a lower extremity revascularization procedure due to symptomatic PAD. 1.9 Treatment of Venous Thromboembolism and Reduction in Risk of Recurrent Venous Thromboembolism in Pediatric Patients XARELTO is indicated for the treatment of venous thromboembolism (VTE) and the reduction in the risk of recurrent VTE in pediatric patients from birth to less than 18 years after at least 5 days of initial parenteral anticoagulant treatment. 1.10 Thromboprophylaxis in Pediatric Patients with Congenital Heart Disease after the Fontan Procedure XARELTO is indicated for thromboprophylaxis in pediatric patients aged 2 years and older with congenital heart disease who have undergone the Fontan procedure.
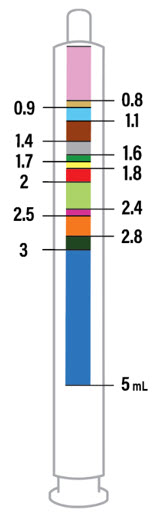
Dosage and Administration
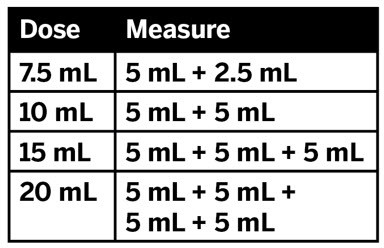
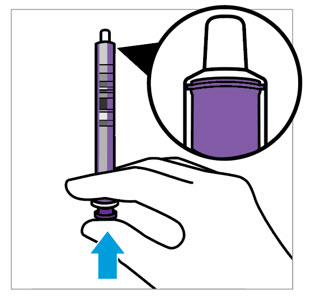
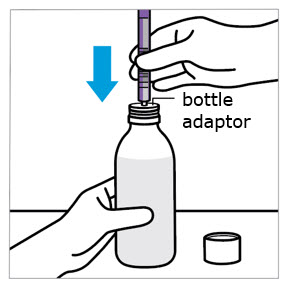
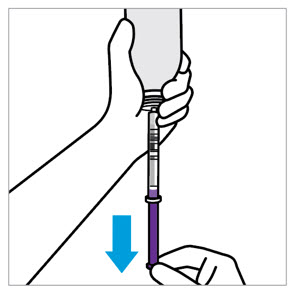
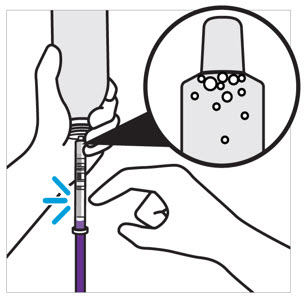
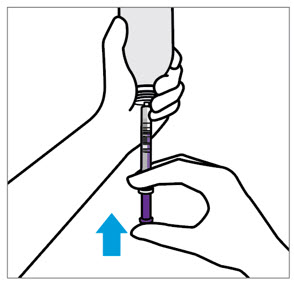
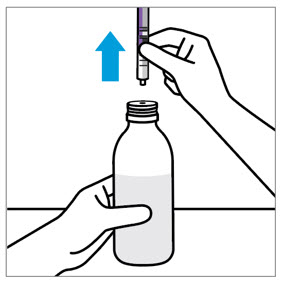
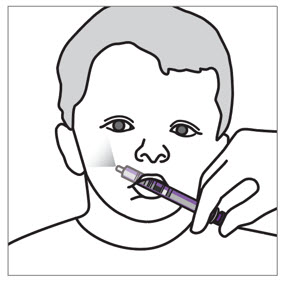
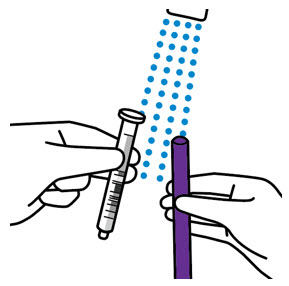
2 DOSAGE AND ADMINISTRATION Nonvalvular Atrial Fibrillation : 15 or 20 mg, once daily with food ( 2.1 ) Treatment of DVT and/or PE : 15 mg orally twice daily with food for the first 21 days followed by 20 mg orally once daily with food for the remaining treatment ( 2.1 ) Reduction in the Risk of Recurrence of DVT and/or PE in patients at continued risk for DVT and/or PE : 10 mg once daily with or without food, after at least 6 months of standard anticoagulant treatment ( 2.1 ) Prophylaxis of DVT Following Hip or Knee Replacement Surgery : 10 mg orally once daily with or without food ( 2.1 ) Prophylaxis of VTE in Acutely Ill Medical Patients at Risk for Thromboembolic Complications Not at High Risk of Bleeding : 10 mg once daily, with or without food, in hospital and after hospital discharge for a total recommended duration of 31 to 39 days ( 2.1 ) CAD or PAD : 2.5 mg orally twice daily with or without food, in combination with aspirin (75–100 mg) once daily ( 2.1 ) Pediatric Patients: See dosing recommendations in the Full Prescribing Information ( 2.2 ) 2.1 Recommended Dosage in Adults Table 1: Recommended Dosage in Adults Indication Renal Considerations Calculate CrCl based on actual weight. [See Warnings and Precautions (5.4) and Use in Specific Populations (8.6)] Dosage Food/Timing See Clinical Pharmacology (12.3) Reduction in Risk of Stroke in Nonvalvular Atrial Fibrillation CrCl >50 mL/min 20 mg once daily Take with evening meal CrCl ≤50 mL/min Patients with CrCl <30 mL/min were not studied, but administration of XARELTO is expected to result in serum concentrations of rivaroxaban similar to those in patients with moderate renal impairment (CrCl 30 to <50 mL/min) [see Use in Specific Populations (8.6)] 15 mg once daily Take with evening meal Treatment of DVT and/or PE CrCl ≥15 mL/min 15 mg twice daily ▼ after 21 days, transition to ▼ 20 mg once daily Take with food, at the same time each day CrCl <15 mL/min Avoid Use Reduction in the Risk of Recurrence of DVT and/or PE in patients at continued risk for DVT and/or PE CrCl ≥15 mL/min 10 mg once daily, after at least 6 months of standard anticoagulant treatment Take with or without food CrCl <15 mL/min Avoid Use Prophylaxis of DVT Following: Hip Replacement Surgery See Dosage and Administration (2.4) CrCl ≥15 mL/min 10 mg once daily for 35 days, 6–10 hours after surgery once hemostasis has been established Take with or without food CrCl <15 mL/min Avoid Use Knee Replacement Surgery CrCl ≥15 mL/min 10 mg once daily for 12 days, 6–10 hours after surgery once hemostasis has been established Take with or without food CrCl <15 mL/min Avoid Use Prophylaxis of VTE in Acutely Ill Medical Patients at Risk for Thromboembolic Complications Not at High Risk of Bleeding CrCl ≥15 mL/min 10 mg once daily, in hospital and after hospital discharge, for a total recommended duration of 31 to 39 days Take with or without food CrCl <15 mL/min Avoid Use Reduction of Risk of Major Cardiovascular Events (CV Death, MI, and Stroke) in CAD No dose adjustment needed based on CrCl 2.5 mg twice daily , plus aspirin (75–100 mg) once daily Take with or without food Reduction of Risk of Major Thrombotic Vascular Events in PAD, Including Patients after Lower Extremity Revascularization due to Symptomatic PAD No dose adjustment needed based on CrCl 2.5 mg twice daily , plus aspirin (75–100 mg) once daily. When starting therapy after a successful lower extremity revascularization procedure, initiate once hemostasis has been established. Take with or without food 2.2 Recommended Dosage in Pediatric Patients Treatment of Venous Thromboembolism and Reduction in Risk of Recurrent Venous Thromboembolism in Pediatric Patients Table 2: Recommended Dosage in Pediatric Patients Birth to Less than 18 Years for Treatment of and Reduction in Risk of Recurrent VTE Initiate XARELTO treatment following at least 5 days of initial parenteral anticoagulation therapy. , Patients <6 months of age should meet the following criteria: at birth were at least 37 weeks of gestation, have had at least 10 days of oral feeding, and weigh ≥2.6 kg at the time of dosing. Dosage Form Body Weight 1 mg XARELTO = 1 mL Suspension Dosage Total Daily Dose All doses should be taken with feeding or with food since exposures match that of 20 mg daily dose in adults. Once a Day Once a day: approximately 24 hours apart; 2 times a day: approximately 12 hours apart; 3 times a day: approximately 8 hours apart 2 Times a Day 3 Times a Day Oral Suspension Only 2.6 kg to 2.9 kg 0.8 mg 2.4 mg 3 kg to 3.9 kg 0.9 mg 2.7 mg 4 kg to 4.9 kg 1.4 mg 4.2 mg 5 kg to 6.9 kg 1.6 mg 4.8 mg 7 kg to 7.9 kg 1.8 mg 5.4 mg 8 kg to 8.9 kg 2.4 mg 7.2 mg 9 kg to 9.9 kg 2.8 mg 8.4 mg 10 kg to 11.9 kg 3 mg 9 mg 12 kg to 29.9 kg 5 mg 10 mg Oral Suspension or Tablets 30 kg to 49.9 kg 15 mg 15 mg ≥50 kg 20 mg 20 mg Dosing of XARELTO was not studied and therefore dosing cannot be reliably determined in the following patient populations. Its use is therefore not recommended in children less than 6 months of age with any of the following: Less than 37 weeks of gestation at birth Less than 10 days of oral feeding Body weight of less than 2.6 kg. To increase absorption, all doses should be taken with feeding or with food. Monitor the child's weight and review the dose regularly, especially for children below 12 kg. This is to ensure a therapeutic dose is maintained. All pediatric patients (except <2 years old with catheter-related thrombosis): Therapy with XARELTO should be continued for at least 3 months in children with thrombosis. Treatment can be extended up to 12 months when clinically necessary. The benefit of continued therapy beyond 3 months should be assessed on an individual basis taking into account the risk for recurrent thrombosis versus the potential risk of bleeding. Pediatric patients <2 years old with catheter-related thrombosis: Therapy with XARELTO should be continued for at least 1 month in children less than 2 years old with catheter-related thrombosis. Treatment can be extended up to 3 months when clinically necessary. The benefit of continued therapy beyond 1 month should be assessed on an individual basis taking into account the risk for recurrent thrombosis versus the potential risk of bleeding. Thromboprophylaxis in Pediatric Patients with Congenital Heart Disease after the Fontan Procedure Table 3: Recommended Dosage for Thromboprophylaxis in Pediatric Patients with Congenital Heart Disease Dosage Form Body Weight 1 mg XARELTO = 1 mL Suspension Dosage Total Daily Dose All doses can be taken with or without food since exposures match that of 10 mg daily dose in adults. Once a Day Once a day: approximately 24 hours apart; 2 times a day: approximately 12 hours apart. 2 Times a Day Oral Suspension Only 7 kg to 7.9 kg 1.1 mg 2.2 mg 8 kg to 9.9 kg 1.6 mg 3.2 mg 10 kg to 11.9 kg 1.7 mg 3.4 mg 12 kg to 19.9 kg 2 mg 4 mg 20 kg to 29.9 kg 2.5 mg 5 mg 30 kg to 49.9 kg 7.5 mg 7.5 mg Oral Suspension or Tablets ≥50 kg 10 mg 10 mg Administration in Pediatric Patients Food Effect: For the treatment of VTE in children, the dose should be taken with food to increase absorption. For thromboprophylaxis after Fontan procedure, the dose can be taken with or without food. Vomit or Spit up: If the patient vomits or spits up the dose within 30 minutes after receiving the dose, a new dose should be given. However, if the patient vomits more than 30 minutes after the dose is taken, the dose should not be re-administered and the next dose should be taken as scheduled. If the patient vomits or spits up the dose repeatedly, the caregiver should contact the child's doctor right away. Tablets: XARELTO tablet must not be split in an attempt to provide a fraction of a tablet dose. For children unable to swallow 10, 15, or 20 mg whole tablets, XARELTO oral suspension should be used. XARELTO 2.5 mg tablets are not recommended for use in pediatric patients [see Use in Specific Populations (8.4) ] . Use in Renal Impairment in Pediatric Patients Patients 1 Year of Age or Older Mild renal impairment (eGFR: 50 to ≤ 80 mL/min/1.73 m 2 ): No dose adjustment is required. Moderate or severe renal impairment (eGFR: <50 mL/min/1.73 m 2 ): avoid use, as limited clinical data are available. Estimated glomerular filtration rate (eGFR) can be done using the updated Schwartz formula, eGFR (Schwartz) = (0.413 × height in cm)/serum creatinine in mg/dL, if serum creatinine (SCr) is measured by an enzymatic creatinine method that has been calibrated to be traceable to isotope dilution mass spectrometry (IDMS). If SCr is measured with routine methods that have not been recalibrated to be traceable to IDMS (e.g., the traditional Jaffé reaction), the eGFR should be obtained from the original Schwartz formula: eGFR (mL/min/1.73 m 2 ) = k * height (cm)/SCr (mg/dL), where k is proportionality constant: k = 0.55 in children 1 year to 13 years k = 0.55 in girls > 13 and < 18 years k = 0.70 in boys > 13 and < 18 years Patients Less than 1 Year of Age Determine renal function using serum creatinine. Avoid use of XARELTO in pediatric patients younger than 1 year with serum creatinine results above 97.5 th percentile, as no clinical data are available. Table 4: Reference Values of Serum Creatinine in Pediatric Patients <1 Year of Age Age 97.5 th Percentile of Creatinine (mg/dL) 97.5 th Percentile of Creatinine (µmol/L) Week 2 0.52 46 Week 3 0.46 41 Week 4 0.42 37 Month 2 0.37 33 Month 3 0.34 30 Month 4–6 0.34 30 Month 7–9 0.34 30 Month 10–12 0.36 32 2.3 Switching to and from XARELTO Switching from Warfarin to XARELTO - When switching patients from warfarin to XARELTO, discontinue warfarin and start XARELTO as soon as the International Normalized Ratio (INR) is below 3.0 in adults and below 2.5 in pediatric patients to avoid periods of inadequate anticoagulation. Switching from XARELTO to Warfarin – Adults: No clinical trial data are available to guide converting patients from XARELTO to warfarin. XARELTO affects INR, so INR measurements made during coadministration with warfarin may not be useful for determining the appropriate dose of warfarin. One approach is to discontinue XARELTO and begin both a parenteral anticoagulant and warfarin at the time the next dose of XARELTO would have been taken. Pediatric Patients: To ensure adequate anticoagulation during the transition from XARELTO to warfarin, continue XARELTO for at least 2 days after the first dose of warfarin. After 2 days of co-administration, an INR should be obtained prior to the next scheduled dose of XARELTO. Co-administration of XARELTO and warfarin is advised to continue until the INR is ≥ 2.0. Once XARELTO is discontinued, INR testing may be done reliably 24 hours after the last dose. Switching from XARELTO to Anticoagulants other than Warfarin - For adult and pediatric patients currently taking XARELTO and transitioning to an anticoagulant with rapid onset, discontinue XARELTO and give the first dose of the other anticoagulant (oral or parenteral) at the time that the next XARELTO dose would have been taken [see Drug Interactions (7.4) ] . Switching from Anticoagulants other than Warfarin to XARELTO - For adult and pediatric patients currently receiving an anticoagulant other than warfarin, start XARELTO 0 to 2 hours prior to the next scheduled administration of the drug (e.g., low molecular weight heparin or non-warfarin oral anticoagulant) and omit administration of the other anticoagulant. For unfractionated heparin being administered by continuous infusion, stop the infusion and start XARELTO at the same time. 2.4 Discontinuation for Surgery and other Interventions If anticoagulation must be discontinued to reduce the risk of bleeding with surgical or other procedures, XARELTO should be stopped at least 24 hours before the procedure to reduce the risk of bleeding [see Warnings and Precautions (5.2) ] . In deciding whether a procedure should be delayed until 24 hours after the last dose of XARELTO, the increased risk of bleeding should be weighed against the urgency of intervention. XARELTO should be restarted after the surgical or other procedures as soon as adequate hemostasis has been established, noting that the time to onset of therapeutic effect is short [see Warnings and Precautions (5.1) ] . If oral medication cannot be taken during or after surgical intervention, consider administering a parenteral anticoagulant. 2.5 Missed Dose Adults For patients receiving 2.5 mg twice daily: if a dose is missed, the patient should take a single 2.5 mg XARELTO dose as recommended at the next scheduled time. For patients receiving 15 mg twice daily: The patient should take XARELTO immediately to ensure intake of 30 mg XARELTO per day. Two 15 mg tablets may be taken at once. For patients receiving 20 mg, 15 mg or 10 mg once daily: The patient should take the missed XARELTO dose immediately. The dose should not be doubled within the same day to make up for a missed dose. Pediatric Patients If XARELTO is taken once a day, the patient should take the missed dose as soon as possible once it is noticed, but only on the same day. If this is not possible, the patient should skip the dose and continue with the next dose as prescribed. The patient should not take two doses to make up for a missed dose. If XARELTO is taken two times a day, the patient should take the missed morning dose as soon as possible once it is noticed. A missed morning dose may be taken together with the evening dose. A missed evening dose can only be taken in the same evening. If XARELTO is taken three times a day, if a dose is missed, the patient should skip the missed dose and go back to the regular dosing schedule at the usual time without compensating for the missed dose. On the following day, the patient should continue with their regular regimen. 2.6 Administration Options For adult patients who are unable to swallow whole tablets, XARELTO tablets (all strengths) may be crushed and mixed with applesauce immediately prior to use and administered orally. After the administration of a crushed XARELTO 15 mg or 20 mg tablet, the dose should be immediately followed by food. Administration with food is not required for the 2.5 mg or 10 mg tablets [see Clinical Pharmacology (12.3) ] . Administration of XARELTO tablets via nasogastric (NG) tube or gastric feeding tube: After confirming gastric placement of the tube, XARELTO tablets (all strengths) may be crushed and suspended in 50 mL of water and administered via an NG tube or gastric feeding tube. Since rivaroxaban absorption is dependent on the site of drug release, avoid administration of XARELTO distal to the stomach which can result in reduced absorption and thereby, reduced drug exposure. After the administration of a crushed XARELTO 15 mg or 20 mg tablet, the dose should then be immediately followed by enteral feeding. Enteral feeding is not required following administration of the 2.5 mg or 10 mg tablets [see Clinical Pharmacology (12.3) ] . Crushed XARELTO tablets (all strengths) are stable in water and in applesauce for up to 4 hours. An in vitro compatibility study indicated that there is no adsorption of rivaroxaban from a water suspension of a crushed XARELTO tablet to PVC or silicone nasogastric (NG) tubing. Administration of XARELTO suspension via NG tube or gastric feeding tube : XARELTO oral suspension may be given through NG or gastric feeding tube. After the administration, flush the feeding tube with water. For the treatment or reduction in risk of recurrent VTE in pediatric patients, the dose should then be immediately followed by enteral feeding to increase absorption. For the thromboprophylaxis in pediatric patients with congenital heart disease who have undergone the Fontan procedure, the dose does not require to be followed by enteral feeding. An in vitro compatibility study indicated that XARELTO suspension can be used with PVC, polyurethane or silicone NG tubing. 2.7 Preparation Instructions for Pharmacy of XARELTO for Oral Suspension Do not add flavor as product is already flavored (sweet and creamy). Reconstitute before dispensing: Tap the bottle until all granules flow freely. Add 150 mL of purified water for reconstitution. Shake for 60 seconds. Check that all granules are wetted and the suspension is uniform. Push the adaptor into bottleneck and recap bottle. The suspension must be used within 60 days. Write the "Discard after" date on the bottle and carton. Dispensing Instructions: Dispense in the original bottle. Dispense the bottle upright with the syringes provided in the original carton. Store reconstituted suspension at room temperature between 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F). Do not freeze. It is recommended the pharmacist counsel the caregiver on proper use. Alert the patient or caregiver to read the Medication Guide and Instructions for Use.
